3.2.2 Electromagmetic radiation and quantum phenomena
3.2.2.1 The photoelectric effect
The photoelectric effect
- Photoelectrons are emitted from the surface of a metal after light above a certain frequency (threshold frequency) is shone on it
Work function (\(\phi\))
- The minimum energy to remove an electron from the metal surface when the metal is at zero potential
Stopping potential (\(V_{s}\))
- The PD needed to apply across the metal to stop the photoelectrons with the maximum KE (\(E_{k(max)}\))
- Minimum energy needed per unit charge to stop photoelectric emissions
- \(E_{k(max)} = e \times V_{s}\)
Threshold frequency
- The minimum frequency of the radiation / light / photon needed to liberate an electron from the surface of a material
- \(hf > \phi\)
- \(f_{min} = \frac{\phi}{h}\)
Why wave theory doesn't work
- There is no photoemission below the threshold frequency even with bright light
- Wave theory would allow gradual accumulation of energy to cause emission
- Any frequency of light should be able to cause electron emission
- Electrons are emitted with no noticeable decay
- In wave theory time would elapse while an electron gains sufficient energy to leave the surface
- Intensity of the light does not affect the KE of the emitted electrons
- High intensity waves would be expected to give higher KE to an electron
Explanation with the photon model
- When light is incident on a metal surface an electron at the surface absorbs a single photon from the incident light and gains energy equal to \(hf\)
- An electron can leave the metal surface if the energy gained > the work function of the metal
- Excess energy gained becomes KE of the photoelectron
Effect of increasing the intensity of light
- There are more photons striking the surface per second
- Current increases as the number of electrons emitted per second increases
Photoelectric equation
- \(E = hf = \phi + E_{k(max)}\)
- \(E_{k(max)} = hf - \phi\)
- Graph
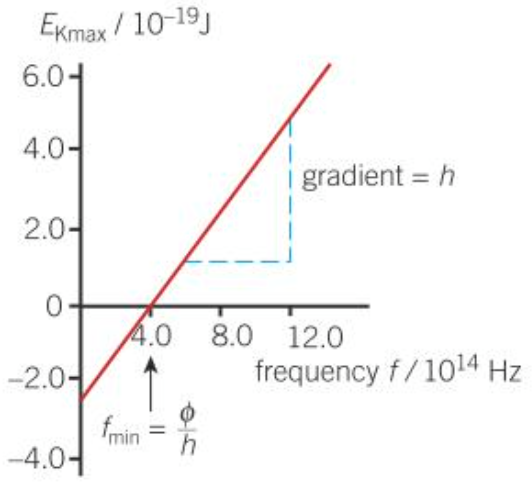
- Gradient = \(h\)
- y-intercept = \(-\phi\)
Energy level of emitted electrons
- There exists a maximum value of energy
- Energy of photons are constant (\(hf\))
- One to one interaction between photon and electron so a fixed amount of KE is transferred
- The energy required to remove an electron varies so the KE of electrons varies
- Max KE = photon energy - work function
- Deeper electrons require more energy to remove than surface electrons
3.2.2.2 Collisions of electrons with atoms
Electron energy level
- Electrons in atoms can only exist in discrete energy levels
- These electrons can gain energy from collisions with free electrons / absorbing photons
Excitation
- Electrons move up in energy level
- It will quickly return to its original energy level (the ground state) and release energy gained as photons
Ionisation
- Electrons gain enough energy to be removed from the atom entirely
- Occurs if the energy of the free electron is greater than the ionisation energy
Excitation energies
- The energy values at which an atom absorbs energy
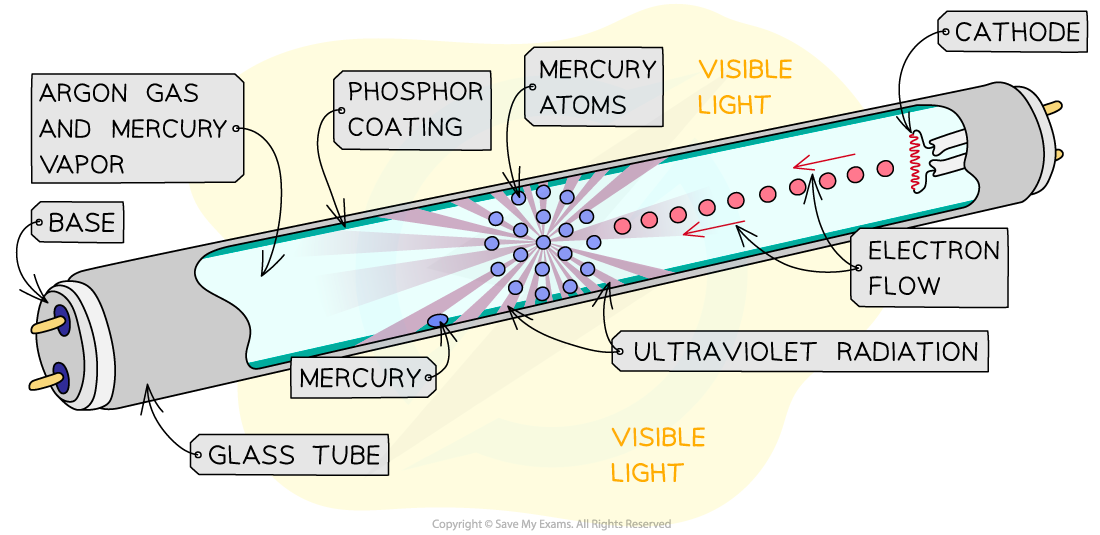
Fluorescent tube
- Filled with mercury vapour
- High voltage applied which accelerates free electrons through the tube
- Free electrons collide with the mercury atoms
- Electrons in the mercury atoms are raised to a higher energy level
- The mercury atom become ionised \(\rightarrow\) release more free electrons
- The new free electrons collide with the mercury atoms, causing them to become excited
- Mercury atoms de-excites and relaxes to a lower energy level
- They release photons of energy equal to the energy difference between the levels
- Frequency is mostly in the UV range
- The fluorescent coating on the inside of the tube absorbs these UV photons and therefore electrons in the atoms of the coating become excited and de-excite releasing photons of visible light
- Emitted radiation consists of (a range of) lower photon energies / frequencies or longer wavelengths
Why electrons only need a minimum energy level to excite an atom
- An exact amount of energy is needed to excite an atom to a certain energy level
- All of the photon's energy will be absorbed in a 1 to 1 interaction
- Electron can transfer part of its energy to cause the excitation and continue moving at a lower KE
3.2.2.3 Energy levels and photon emission
Ground state
- When electrons / atoms are in there lowest energy state / most stable state
Excited state
- Electron (in ground state) has moved to higher energy level / shell
Ionisation energy
- The minimum energy to remove an electron from an atom from the ground state
De-excitation
- The electron configuration in an excited atom is unstable due to a vacancy in the shell that the excited electron left
- The vacancy is filled by an electron from an outer shell transferring to it
Possible energy level of atoms
- An atom can only have certain levels of energy
- Each allowed energy level = a certain electron configuration of the atom
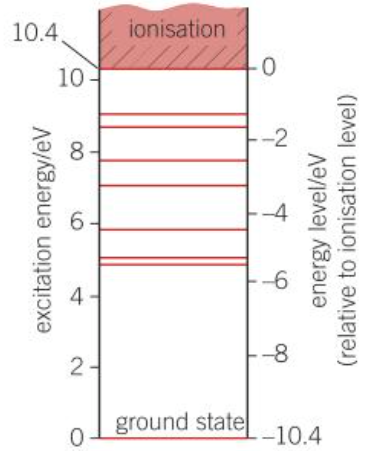
Line spectrum
- Obtained by passing the light from a fluorescent tube through a diffraction grating or prism
- Each line = a specific wavelength of light emitted by the tube = corresponds a specific photon energy emitted
- Show that electrons in atoms can only transition between discrete energy levels

Line absorption spectrum
- Continuous spectrum with black lines at certain intervals
- Obtained by passing white light through a cooled gas
- Black lines represent the possible differences in energy levels
- The atoms in the gas can only absorb photons of an energy equal to the exact difference between two energy levels
Why only certain frequencies of light can be absorbed
- Electrons occupy discrete energy levels
- They need to absorb an exact amount of energy to move to a higher level
- Photons need to have certain frequency to provide this energy (\(E=hf\))
- Energy required is the same for a particular atom
- All energy of the photon is absorbed in 1 to 1 interaction between photon + electron
Energy level difference
- Difference between two energy levels = a line in the line spectrum = a specific photon energy emitted by a fluorescent tube / absorbed in a line absorption spectrum
- Energy of photon emitted = energy lost by the electron = energy lost by the atom
- Energy of the emitted photon \(hf = E_{1} - E_{2}\)
3.2.2.4 Wave-particle duality
Evidence for wave-particle duality of light / EM waves
- Acting as wave: diffraction and interference
- Acting as particle: photoelectric effect
De Broglie hypothesis
- Matter particles have a dual wave-particle nature
Evidence for de Broglie hypothesis
- Collisions by incident electrons move electrons in atoms between energy levels
- Photon emitted when atoms de-excite or electrons move to lower energy levels
- Wave properties of electrons
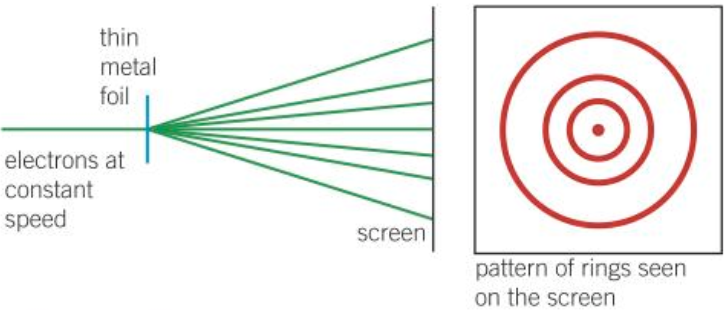
- Diffraction of electrons by a metal crystal
- Electrons can be diffracted, shown as concentric rings on screen
- Foil / graphite causes electrons to travel in particular directions
- Bright rings / maximum intensity occurs where waves arrive in phase and interfere constructively
- Particle behaviour would only produce a circle of light as particles scatter randomly
- Only waves can experience diffraction \(\rightarrow\) electrons also have a dual wave-particle nature
- Similar to diffracting grating maxima when \(n \lambda = d \sin \theta\)
- Particle properties of electrons
- Electrons must provide enough kinetic energy for light to be emitted
- Instant light as electron can provide the energy in discrete amounts to excite the atoms
- Waves \(\rightarrow\) energy will accumulate gradually so time is needed until light is emitted & light will always be emitted no matter how low the energy is
- Property later also shown for other particles
De Broglie wavelength
- The wavelength of the wave-like behaviour of a matter particle
- \(\lambda = \frac{h}{p} = \frac{h}{mv}\)
- Higher particle momentum = shorter wavelength = less diffraction = concentric rings of the interference pattern become closer
Change in understanding of matter
- Knowledge and understanding of the nature of matter changes over time in line with new experimental evidence gathered
- Such changes need to be evaluated through peer review and validated by the scientific community before being accepted