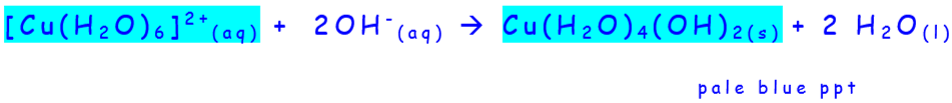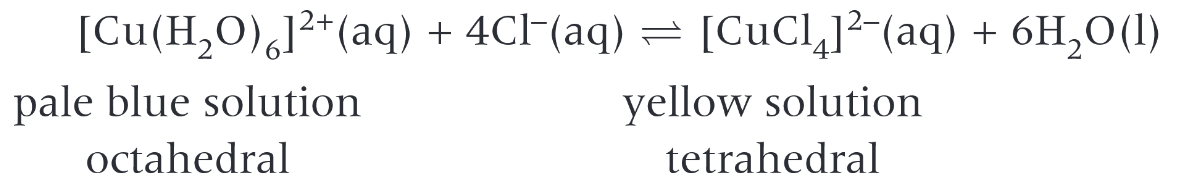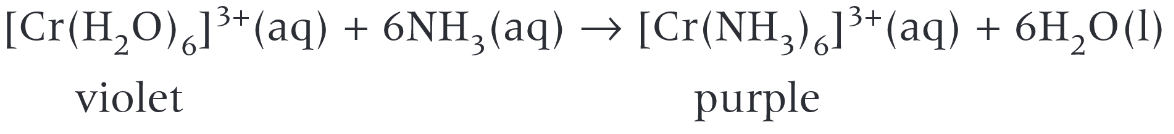5.3.1 Transition elements
Definitions
| Term | Definition |
|---|---|
| Transition element | A d-block element that has an ion with an incomplete d sub-shell |
| Complex ion | A transition metal ion bonded to one or more ligands |
| Ligand | A molecule or ion that donates a pair of electrons to a central metal ion to form a coordinate bond |
| Coordination number | The number of dative bonds the transition metal ion has to its ligands |
| Monodentate ligands | Donate one pair of electrons to a central metal ion e.g. \(H_2O\), \(Cl^-\), \(NH_3\) |
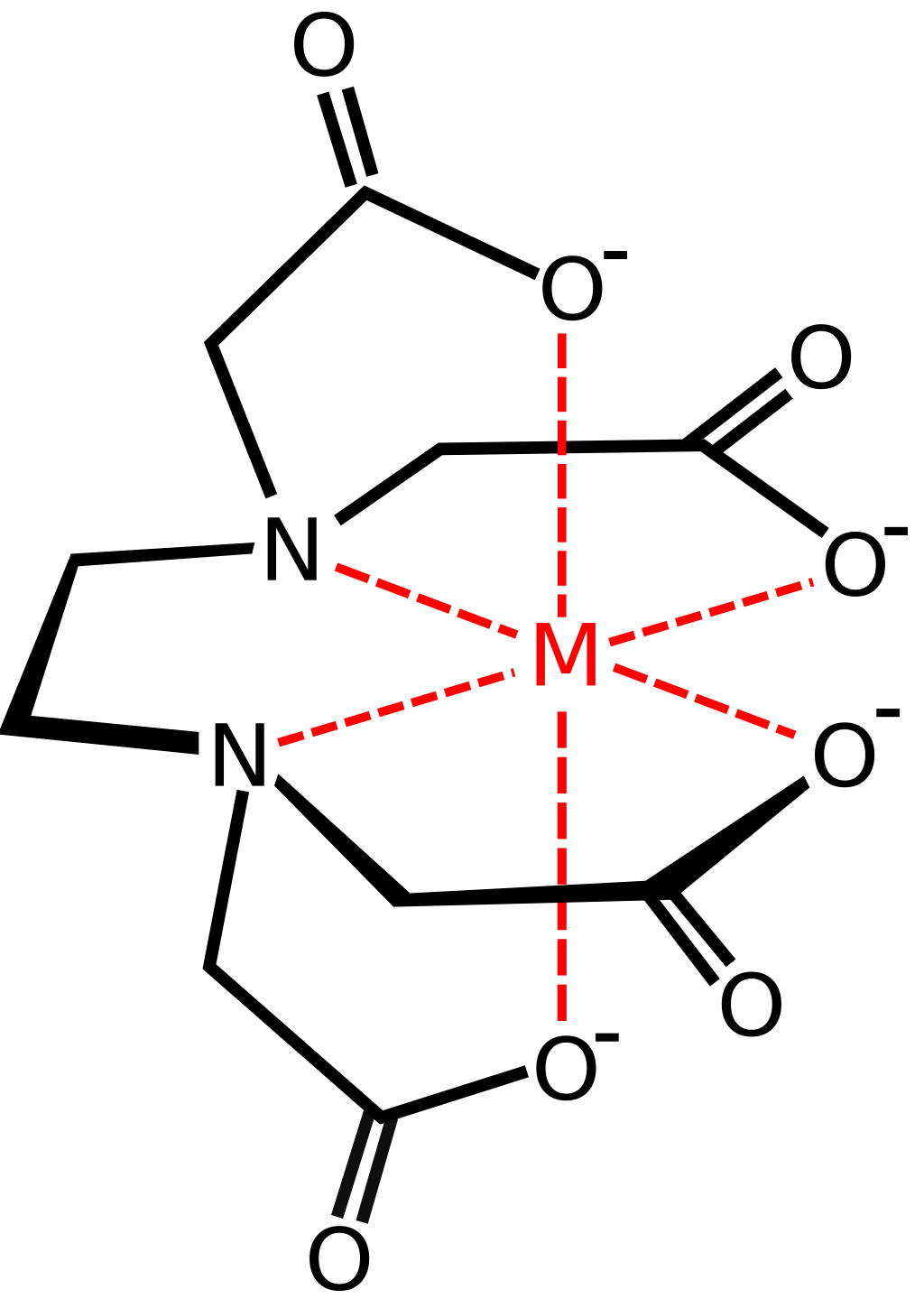
| Bidentate ligands | Donate two pairs of electrons to the central metal ion e.g. \(NH_2CH_2CH_2NH_2\) (shortened to en), ethanedioate |
| Optical isomers | Non-superimposable mirror images of each other |
Properties
Electron configuration of period 4 atoms (Sc → Zn)
- Highest energy subshell=3d, electrons are added to 3d
| Element | Number of electrons | Electron configuration |
|---|---|---|
| Scandium | 21 | \([Ar]3d^1 4s^2\) |
| Titanium | 22 | \([Ar]3d^2 4s^2\) |
| Vanadium | 23 | \([Ar]3d^3 4s^2\) |
| Chromium | 24 | \([Ar]3d^5 4s^1\) |
| Manganese | 25 | \([Ar]3d^5 4s^2\) |
| Iron | 26 | \([Ar]3d^6 4s^2\) |
| Cobalt | 27 | \([Ar]3d^7 4s^2\) |
| Nickel | 28 | \([Ar]3d^8 4s^2\) |
| Copper | 29 | \([Ar]3d^{10} 4s^1\) |
| Zinc | 30 | \([Ar]3d^{10} 4s^2\) |
- Chromium and copper do not follow the expected pattern
- Half-filled / fully filled d sub-shell gives additional stability
Electron configuration of period 4 ions (Sc → Zn)
- 4s orbital is filled before 3d as it has a lower energy level when unfilled
- The 4s orbital empties before the 3d orbitals as the 3d energy level drops below 4s after it is filled
Exceptions in d-block
- Scandium and zinc are not transition elements
- Scandium only forms \(Sc^{3+}\): \(1s^2 2s^2 2p^6 3s^2 3p^6\) (empty d-sub-shells)
- Zinc only forms \(Zn^{2+}\): \(1s^2 2s^2 2p^6 3s^2 3p^6 3d^{10}\) (full d-sub-shells)
- Their only ions do not have an incomplete d-sub-shell
Properties of transition metals
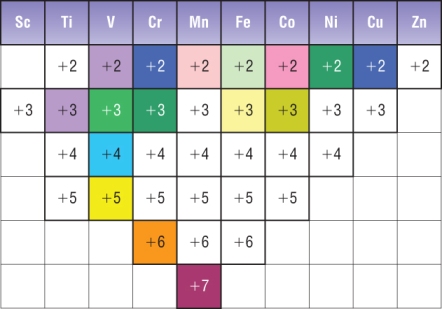
- Form compounds with different oxidation states / multiple positive ions
- Form coloured compounds (dissolve in water to form coloured solutions)
- Elements / compounds can act as catalysts
Oxidation states
- They all form 2+ ions by losing the 4s electrons
- They can then easily lose some / all of the 3d electrons
- Species with transition element in highest oxidation state are often strong oxidising agents
- Often form complex ions in higher oxidation states
Colour of transition elements
- Linked to partially filled d-orbitals, can vary depending on oxidation state
- Potassium dichromate(VI) = bright orange
- Cobalt(II) chloride = pink/purple
- Nickel(II) sulfate = green
- Hydrated copper(II) sulphate = blue
Transition elements as catalysts
- Heterogeneous catalysts are preferred as they are in a different state to reactants so easy to separate
- Allow reactions to carry out at lower temperature and pressure which reduces energy usage
- Benefits of energy savings is often counteracted by the toxicity of many transition metals
Ligands and complex ions
Common ligands
- EDTA (ethylenediaminetetraacetic acid, a hexadentate ligand)
Six-coordinate complexes shapes
- Octahedral shape
- Bond angle 90° around the central metal ion
- e.g. \([Cu(H_2O)_6]^{2+}\), \([Fe(H_2O)_6]^{3+}\), \([Mn(H_2O)_6]^{2+}\)
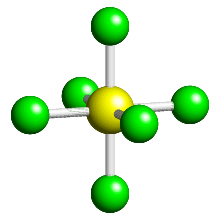
Four-coordinate complexes shapes
- Tetrahedral shape (more common)
- Bond angle 109.5° around the central metal ion
- e.g. \([CuCl_4]^{2-}\), \([CoCl_4]^{2-}\)
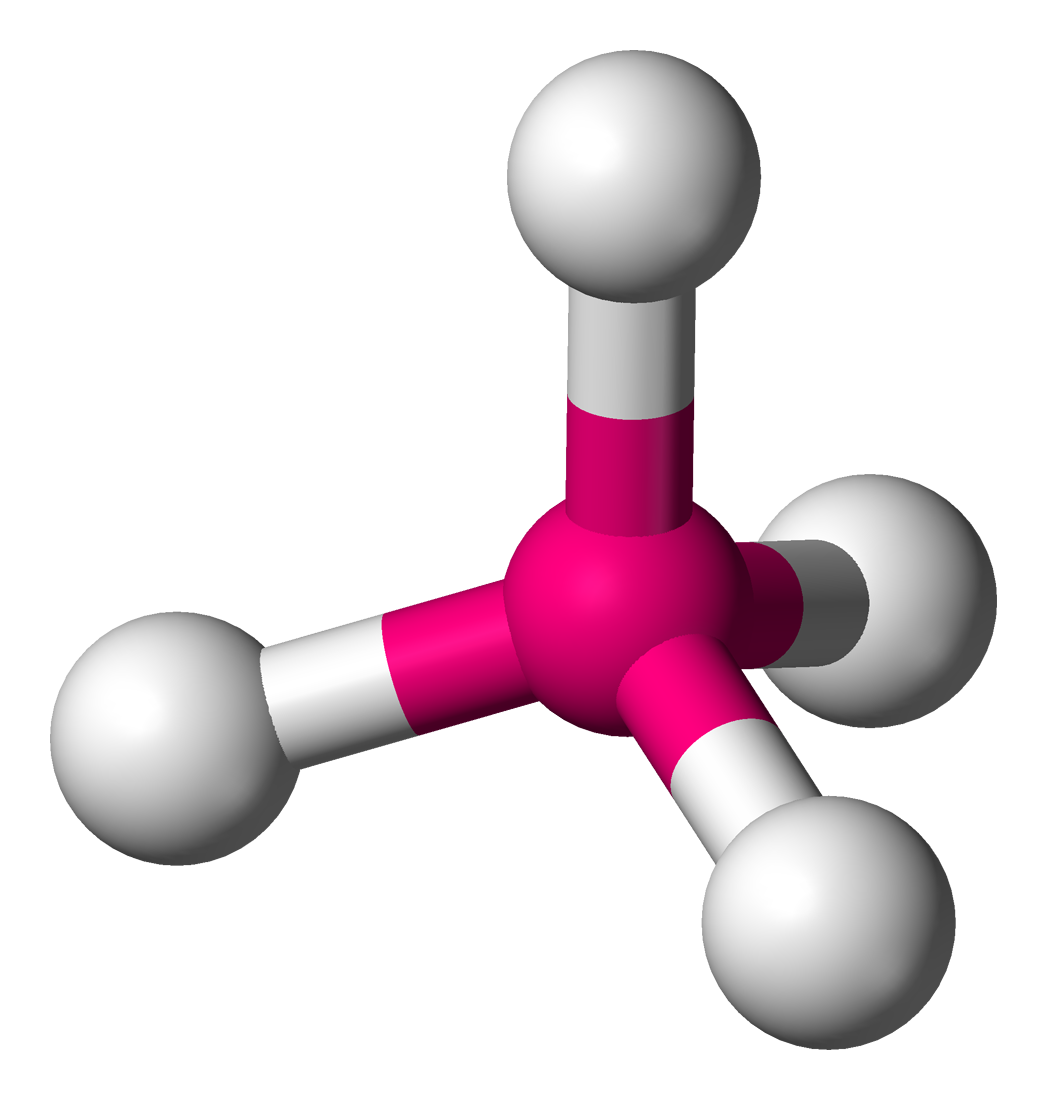
- Square planar shape
- Bond angle 90° around the central metal ion
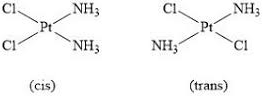
- e.g. complexes of Pt: \([Pt(NH_3)_4]^{2+}\), \([Pt(NH_3)_2Cl_2]\)
Cis-trans isomerism in square planar ions
- No more than 2 identical ligands of each type attached to the central metal ion
- e.g. \([Pt(NH_3)_2Cl_2]\): \(Pt^{2+}\) ion + \(2 \times NH_3\) + \(2 \times Cl^-\)
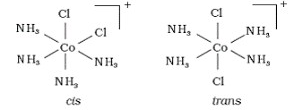
Cis-trans isomerism in octahedral ions
- Cis = identical groups adjacent (90°); trans = identical groups opposite (180°)
- Monodentate ligands only
- 4 of one type of ligand and 2 of another type
- e.g. \([Co(NH_3)_4Cl_2]^+\): cis-isomer = violet, trans-isomer = green
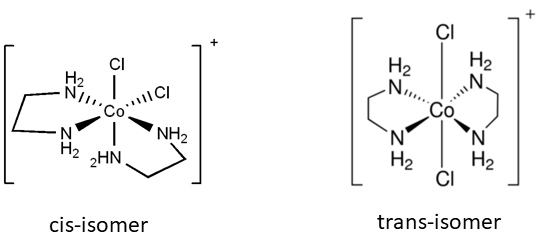
- Bidentate ligands
- 2 bidentate ligands + 2 monodentate ligands
- e.g. \([Co(NH_2CH_2CH_2NH_2)_2Cl_2]^+\)
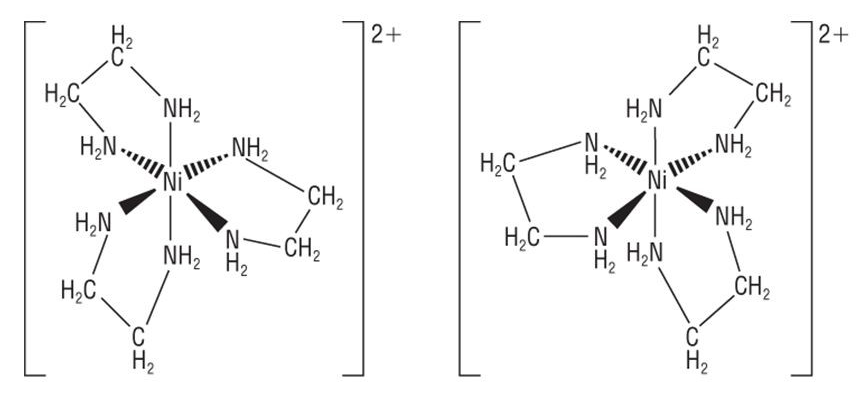
Optical isomerism in octahedral complexes
- 2 or more bidentate ligands
- 2 bidentate ligands: e.g. cis-isomer of \([Co(NH_2CH_2CH_2NH_2)_2Cl_2]^+\)
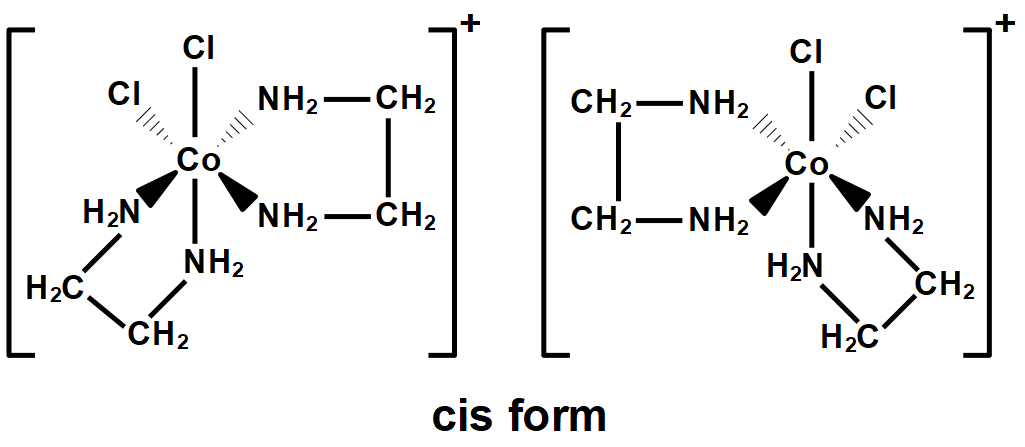
- (Optical isomerism cannot exist for trans-isomers as the mirror image is exactly the same as the original image and can be superimposed)
- 3 bidentate ligands: e.g. \([Ru(NH_2CH_2CH_2NH_2)_3]^{2+}\) / \([Ni(NH_2CH_2CH_2NH_2)_3]^{2+}\)
Use of cis-platin in medicine
- Used as an anti-cancer drug to attack + shrink tumours
- Bind to DNA in cancer cells \(\rightarrow\) preventing cell division
- It forms a platinum complex inside a cell
- An example of chemotherapy
- Drugs used in chemostherapy are always toxic which means that they also lead to side effects
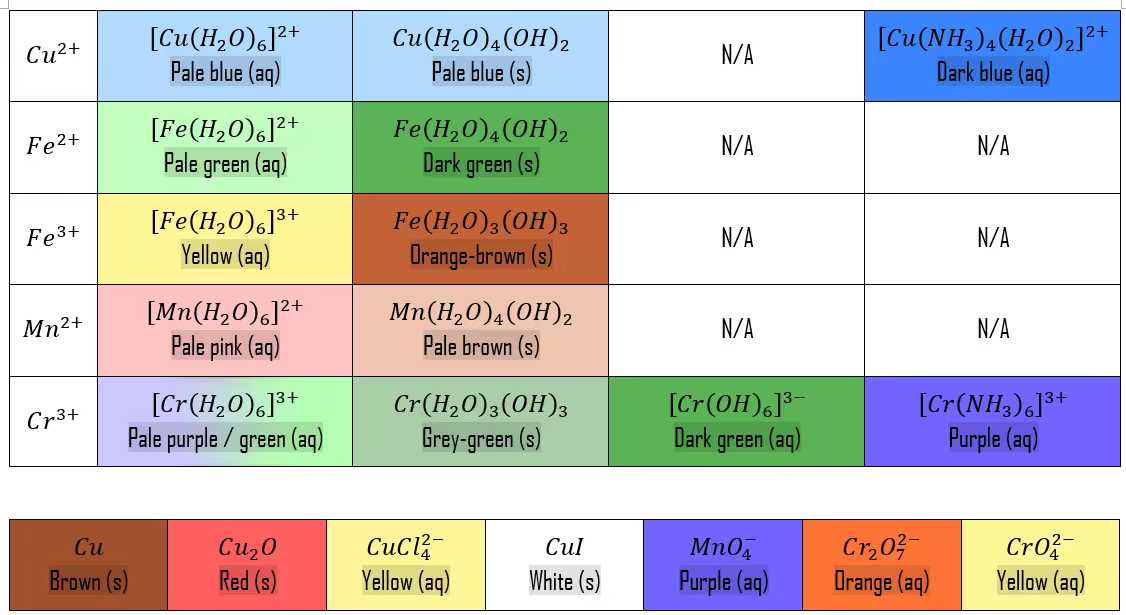
Common complexes
| Complex | Shape | Colour |
|---|---|---|
| \([Cu(H_2O)_6]^{2+}\) | Octahedral | (Pale) blue |
| \([Fe(H_2O)_6]^{3+}\) | Octahedral | Brown |
| \([CuCl_4]^{2-}\) | Tetrahedral | Yellow |
| \(Pt(NH_3)_2Cl_2\) (platin) | Square planar | No colour |
| \([CoCl_4]^{2-}\) | Tetrahedral | Blue |
| \([Co(H_2O)_6]^{2+}\) | Octahedral | Pink |
| \([Cu(NH_3)_4(H_2O)_2]^{2+}\) | Octahedral | Deep blue solution |
| \([Cu(H_2O)_4(OH)_2]^{2+}\) | Octahedral | Pale blue precipitation |
| \([Cr(NH_3)_6]^{3+}\) | Octahedral | Purple |
| \([Cr(H_2O)_6]^{3+}\) | Octahedral | Violet |
| \([Mn(H_2O)_4(OH)_2]^{2+}\) | Octahedral | Light brown |
Ligand substitution
Ligand substitution reaction
- A reaction in which one ligand in a complex ion is replaced by another ligand
- The final outcome mainly depends on which ligand is more abundant
- Sometimes this will result in a change in colour
Common ligand substitution reactions
- Hydroxide ions to copper
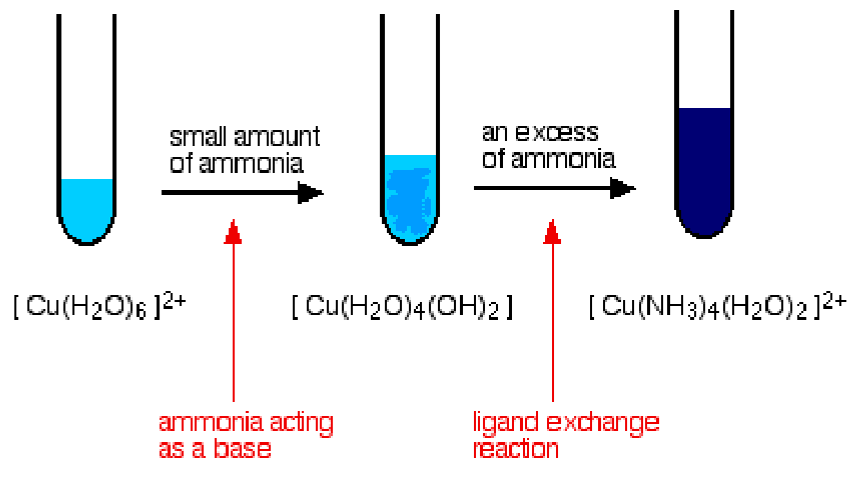
- Ammonia to copper
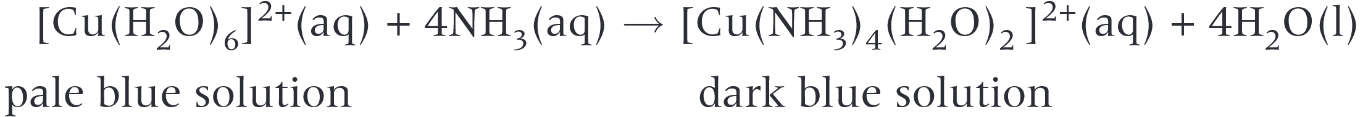
- Ammonia can deprotonate water molecules / substitute them
- 2 step reaction: pale blue precipitate of \(Cu(OH)_2\) formed first which then dissolves in excess ammonia
- Chloride ions to copper
- Ammonia to chromium
Haemoglobin
- Five of the octahedral positions are filled by lone pairs on nitrogen atoms within the protein structure
- The final position can be filled by \(O_2\) / \(CO_2\) / \(H_2O\) / \(CO\)
- Lone pair on oxygen atom forms a coordinate bond to the central \(Fe^{2+}\) ion
- Oxygen bonds to the central \(Fe^{2+}\) ion as blood passes through the lungs due to increased oxygen pressure in capillaries \(\rightarrow\) oxyhaemoglobin forms
- The oxygen is released to body cells when required
- \(CO_2\) binds to haemoglobin and is carried back to the lungs and then released by exhalation
- \(CO\) can also bind to haemoglobin to form carboxyhaemoglobin by replacing oxygen in oxyhaemoglobin
- Carbon monoxide binds more strongly than oxygen so the bond is irreversible
- If carboxyhaemoglobin concentration is too high, oxygen transport is prevented \(\rightarrow\) death
Precipitation reactions
Precipitation reactions
- Occurs when two aqueous solutions containing ions react to form an insoluble ionic solid (precipitate)
Precipitation reactions with NaOH
| Ion | Solution colour | Precipitate colour | Soluble / insoluble in excess NaOH |
|---|---|---|---|
| \(Cu^{2+}\) | Blue | Blue | \(\times\) |
| \(Fe^{2+}\) | Pale green | Green Turns brown on surface in air (\(Fe^{2+} \rightarrow Fe^{3+}\)) |
\(\times\) |
| \(Fe^{3+}\) | Pale yellow | Orange-brown | \(\times\) |
| \(Mn^{2+}\) | Pale pink | Light brown Darkens on standing in air |
\(\times\) |
| \(Cr^{3+}\) | Violet | Grey-green | \(\checkmark \ Cr(OH)_3(s) + 3OH^-(aq) \rightarrow [Cr(OH)_6]^{3-}(aq)\) |
- Overall equations: \(M^{a+}(aq) + aOH^-(aq) \rightarrow M(OH)_a(s)\)
- Can also form complex ions e.g. \([Cu(H_2O)_6]^{2+}\)
Precipitation reactions with ammonia
- In the first stage of ligand substitution reactions with excess ammonia precipitation reaction takes place
- e.g. \(Cu^{2+} \rightarrow Cu(OH)_2\) , \(Cr^{3+} \rightarrow Cr(OH)_3\), same for other ions
- Further reactions: \(Cr(OH)_3\) dissolves to form \([Cr(NH_3)_6]^{3+}\), \(Cu(OH)_2\) dissolves to form \([Cu(NH_3)_4(H_2O)_2]^{2+}\), other precipitates don't react further
Redox reactions
Oxidation of \(Fe^{2+}\) to \(Fe^{3+}\)
- \(MnO_4^-(aq) + 8H^+(aq) + 5Fe^{2+}(aq) \rightarrow Mn^{2+}(aq) + 5Fe^{3+}(aq) + 4H_2O(l)\)
- \(Fe^{2+}\) oxidised to \(Fe^{3+}\), \(MnO_4^-\) reduced to \(Mn^{2+}\)
- In acid conditions
- Used as a basis for redox titration
- Purple from \(MnO_4^-\) \(\rightarrow\) colourless \(Mn^{2+}\)
Reduction of \(Fe^{3+}\) to \(Fe^{2+}\)
- \(2Fe^{3+}(aq) + 2I^-(aq) \rightarrow 2Fe^{2+}(aq) + I_2(aq)\)
- \(Fe^{3+}\) reduced to \(Fe^{2+}\), \(I^-\) oxidised to \(I_2\)
- Orange-brown from \(Fe^{3+}\) \(\rightarrow\) pale-green from \(Fe^{2+}\)
- Colour change obscured by \(I_2\) (brown)
Reduction of \(Cr_2O_7^{2-}\) to \(Cr^{3+}\)
- \(Cr_2O_7^{2-}(aq) + 14H^+(aq) + 3Zn(aq) \rightarrow 2Cr^{3+}(aq) + 7H_2O(l) + 3Zn^{2+}(aq)\)
- Orange from \(Cr_2O_7^{2-}\) \(\rightarrow\) green from \(Cr^{3+}\)
- With an excess of zinc, \(Cr(III)\) ions are reduced further because zinc is a powerful reducing agent
- \(Zn(s) + 2Cr^{3+}(aq) \rightarrow Zn^{2+}(aq) + 2Cr^{2+}(aq)\)
- Green from \(Cr^{3+}\) \(\rightarrow\) pale blue from \(Cr^{2+}\)
Oxidation of \(Cr^{3+}\) to \(CrO_4^{2-}\)
- \(3H_2O_2 + 2Cr^{3+} + 10OH^- \rightarrow 2CrO_4^{2-} + 8H_2O\)
- \(H_2O_2\) is a powerful oxidising agent
Reduction of \(Cu^{2+}\) to \(Cu^+\)
- \(2Cu^{2+}(aq) + 4I^-(aq) \rightarrow 2CuI(s) + I_2(s)\)
- Pale blue from \(Cu^{2+}\) \(\rightarrow\) white precipitate in \(CuI\) + brown \(I_2\)
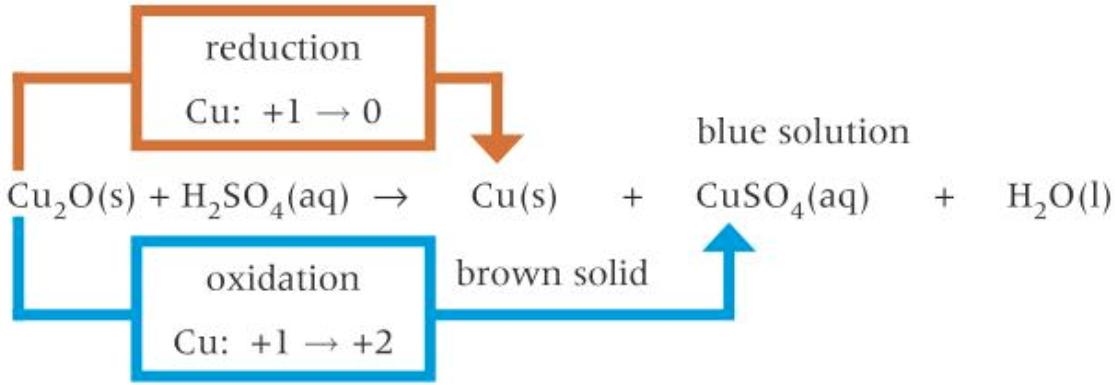
Disproportionation of \(Cu^+\) ions
- \(Cu^+\) readily disproportionates in aqueous conditions