5.2.3 Redox and electrode potentials
Definitions
| Term | Definition |
|---|---|
| Oxidising agent | A reagent that accepts / takes in electrons / oxidises another species / is reduced |
| Reducing agent | A reagent that donates / gives out electrons / reduces another species / is oxidised |
| Standard electrode potential \(E^\ominus\) | The emf of a half-cell compared to a hydrogen electrode under standard conditions |
| Standard half cell | The pure metal in contact with a 1 molar solution of its ions at a temperature of 298 K |
Balancing chemical equations
Balancing equations with missing oxygen
- Acidic condition
- Add 1 \(H_2O\) on the side with less oxygen for every missing oxygen
- Add 2 \(H^+\) on the other side for every \(H_2O\) added
- Alkaline condition
- Add 2 \(OH^-\) on the side with less oxygen for every missing oxygen
- Add 1 \(H_2O\) on the other side for every pair of \(OH^-\) added
- Balance changes in oxidation numbers with electrons
Balancing equations using oxidation numbers
- Sum of oxidation numbers on LHS = sum of oxidation numbers on RHS
- Look at the change in oxidation number of each element
- Multiply chemicals by different amounts so total change = 0
Redox reactions and titrations
Carrying out Manganate(VII) titrations
- Manganate reduced from \(Mn^{7+}\) to \(Mn^{2+}\)
- \(KMnO_4\) is used as the oxidation agent and added to burette
- The reducing agent is added to the conical flask
- Excess dilute \(H_2SO_4\) added to provide \(H^+\) for reduction of \(MnO_4^-\)
- Manganate in the burette is deep purple \(\rightarrow\) reacts and become colourless when added to the flask
- Once all reducing agent has reacted there will be nothing to decolourise the manganate
- End point = when permanent pink colour appears in flask
- \(KMnO_4\) is self indicating so no indicator needed
Iodine / thiosulfate redox titrations
- \(Na_2S_2O_3\) (sodium thiosulfate) reduces iodine to iodide ions and forms \(Na_2S_4O_6\) (sodium tetrathionate)
- \(2S_2O_3^{2-}(aq) + I_2(aq) \rightarrow 2I^-(aq) + S_4O_6^{2-}(aq)\)
- Standard solution of \(Na_2S_2O_3\) added to burette
- An excess of iodide is added to conical flask as iodine is almost insoluble in water but very soluble in iodide solution
- Solution of oxidising agent being analysed is added to conical flask with excess \(KI\)
- Oxidising agent reacts with iodide ions to produce iodine \(\rightarrow\) turn solution yellow-brown
- Iodine reduced back to \(I^-\) ions \(\rightarrow\) brown colour fades gradually
- Indicator is starch: added towards the end when most of the iodine has reacted and gives a blue-black colour
- End point: blue black disappears when iodine is used up and reaction mixture becomes colourless
Electrode potentials
Interpreting electrode potential value
- More positive value: greater tendency to gain electrons + undergo reduction
- More negative value: greater tendency to lose electrons + undergo oxidation
Half cell
- Contains the species present in a redox half equation (forward reaction shows reduction)
- Consists of a piece of pure metal in contact with a solution of its ions
- Electron can be transferred between the metal and its ions
Factors affecting electrode potential of a half cell
- The metal used
- Concentration of the solution of ions
- Temperature
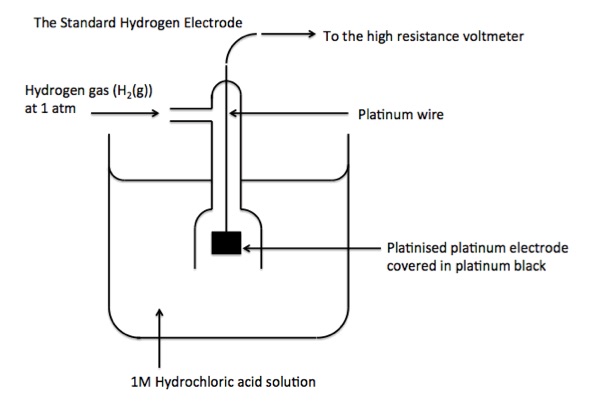
Hydrogen half cell
- Half cell containing \(H_2(g)\) and solution of \(H^+\)
- Inert platinum electrode to allow electrons in and out of the half cell
- Given standard electrode potential value of 0
Measuring standard electrode potentials
- Standard conditions must be used
- Metal ion solutions must have a concentration of \(1 \ mol \cdot dm^{-3}\)
- Gas half cells must be at 100kPa pressure + use inert electrode
- Temperature must be 298K
- Half cell measured connected by wire with a standard hydrogen half cell
- Two solutions connected with a salt bridge to complete the electrical circuit
- Typically contains a concentrated solution of an electrolyte that does not react with either solution (e.g. \(KNO_3\))
- Connect the half-cells to voltmeter (high resistance)
Predicting feasibility of reactions with standard cell potentials
- Strongest oxidising agent = most positive \(E^\ominus\) + most likely to be reduced (will be on right of equation if written as standard form)
- Strongest reducing agent = most negative \(E^\ominus\) + most likely to be oxidised
- (\(X\) system is more negative than \(Y\) system \(\rightarrow\) \(X\) shifts left and \(Y\) shifts right / \(X\) reduces \(Y\))
Limitations of predictions using \(E^\ominus\) values
- Reactions can have very high activation energies \(\rightarrow\) very slow reaction rates
- Actual conditions may not be standard
- Value of electrode potential will be different from standard value if concentration isn’t \(1 \ mol \cdot dm^{-3}\)
- Increasing ion concentration: equilibrium shifts right, removes electrons, \(E^\ominus\) less negative
- Reducing ion concentration: equilibrium shifts left, increases electrons in system, \(E^\ominus\) more negative
- Standard potentials are for aqueous equilibria: many reactions aren’t aqueous
- Value of electrode potential will be different from standard value if concentration isn’t \(1 \ mol \cdot dm^{-3}\)
- There may be an alternative reaction that is more favourable
Storage and fuel cells
Storage cells
- 3 main types
- Primary (non-rechargeable) cells
- Electrical energy produced by an irreversible reaction
- The reactants will be used up eventually and the reaction becomes too slow to create enough voltage
- Used for low current, long-storage devices e.g. clocks or smoke detectors
- Secondary (rechargeable) cells
- Electrical energy produced by a reversible reaction
- The cell can be recharged by reversing cell polarities and forcing electrical energy through the cell
- Cell reaction is reversed during recharging
Lithium-ion/lithium-ion polymer cells
- Can be regular shape or a flexible solid polymer
- Charging / discharging: \(Li^+\) ions move between electrodes, electrons move through connecting wires
- Negative electrode: graphite coated with lithium metal, \(Li \rightarrow Li^+ + e^-\)
- Positive electrode: metal oxide (typically \(CoO_2\)), \(Li^+ + CoO_2 + e^- \rightarrow LiCoO_2\)
Lithium-ion/lithium-ion polymer cells limitations
- Can become unstable at high temperatures
- Can ignite devices
- Hard to recycle since lithium is very reactive
Fuel cells
- Use energy from reaction of fuel with oxygen to create voltage
- A continuous supply of fuel and an oxidising agent (usually oxygen) to the cell
- Fuel supplied to one electrode; oxidising agent to the other electrode
- Can operate continuously if fuel and oxygen are continuously supplied - don’t have to be recharged
Hydrogen-oxygen fuel cells
- Produce only water as combustion product, no \(CO_2\) produced
- Fuel cells using hydrogen rich fuels e.g. methanol are being developed
- Will produce carbon dioxide alongside water
- May produce more pollution but liquid fuel is much easier to store than gaseous ones
- Hydrogen supplied to anode
- Oxidation in anode / negative electrode = oxidation
- Oxygen supplied to cathode
- Reduction in cathode / positive electrode = reduction
Hydrogen fuel cell equations
- Alkaline conditions
- Negative electrode / anode: \(2H_2(g) + 4OH^-(aq) \rightarrow 4H_2O(l) + 4e^-\)
- Positive electrode / cathode: \(O_2(g) + 2H_2O(l) + 4e^- \rightarrow 4OH^-(aq)\)
- Acidic conditions
- Negative electrode / anode: \(2H_2(g) \rightarrow 4H^+(aq) + 4e^-\)
- Positive electrode / cathode: \(O_2(g) + 4H^+(aq) + 4e^- \rightarrow 2H_2O(l)\)