5.2.1 Lattice enthalpy
Definitions
| Term | Definition |
|---|---|
| Lattice enthalpy (\(\Delta_{LE}H\)) | The enthalpy change when one mole of a solid ionic crystal lattice is formed from its constituent gaseous ions (A measure of the strength of ionic bonding in a giant ionic lattice) |
| (First) Electron affinity (\(\Delta_{EA}H\)) | The enthalpy change that takes place when one mole of gaseous atoms gains one mole of electrons to form one mole of gaseous 1- ions |
| Atomisation energy | The enthalpy change required to form 1 mole of gaseous atoms from the element in its standard state |
| Dissociation energy | The energy required to break one mole of a bond in the gas state |
| Enthalpy change of solution (dissolution) (\(\Delta_{sol}H\)) | The enthalpy change when one mole of an ionic solid dissolves in a solvent (to form aqueous ions) |
| Enthalpy change of hydration | The enthalpy change that accompanies the dissolving of one mole of gaseous ions in water to form one mole of aqueous ions |
Born–Haber and related enthalpy cycles
Born-Haber cycle calculation
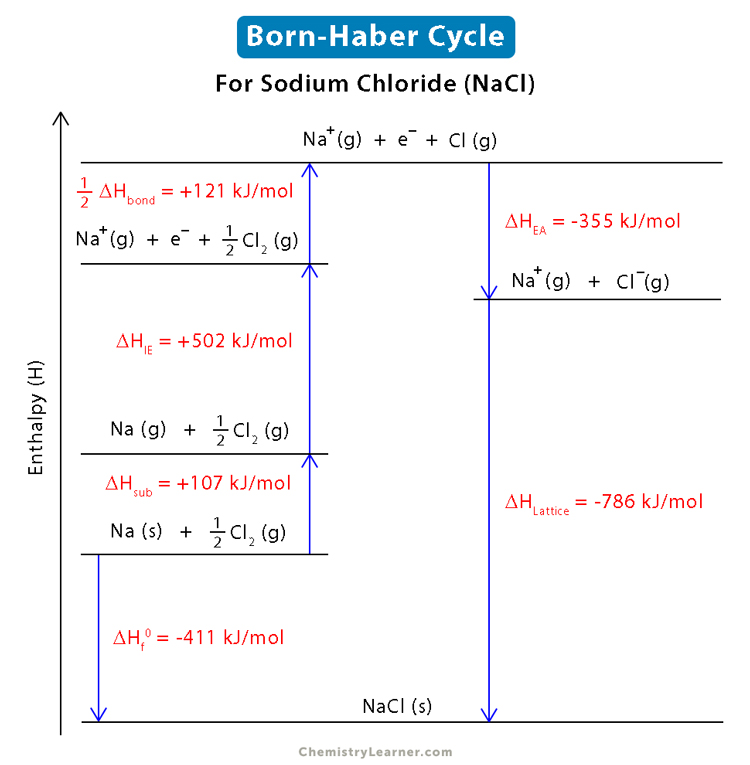
- Lattice enthalpy = enthalpy change of formation - (enthalpy change of atomisation + ionisation energy + electron affinity)
Exothermic / endothermic energy changes
| Always exothermic | Always endothermic | Varies |
|---|---|---|
| - First electron affinity - Lattice enthalpy - Enthalpy change of hydration |
- Atomisation energies - Dissociation energies - All other electron affinity - Ionisation energies |
- Enthalpy change of solution |
Factors affecting enthalpy changes
Factors affecting lattice enthalpy
- Ionic size
- Ionic radius increases \(\rightarrow\) charge density decreases \(\rightarrow\) attraction between ions decreases
- Less exothermic lattice enthalpy
- Lower melting point
- Ionic charge
- Increasing charge = attraction between ions increases
- More exothermic lattice enthalpy
- Higher melting point
Factors affecting enthalpy change of hydration
- Ionic size
- Ionic radius increases \(\rightarrow\) attraction between ion and water molecules decreases
- Hydration energy less exothermic
- Ionic charge
- Ionic charge increases \(\rightarrow\) attraction with water increases
- Hydration energy more exothermic
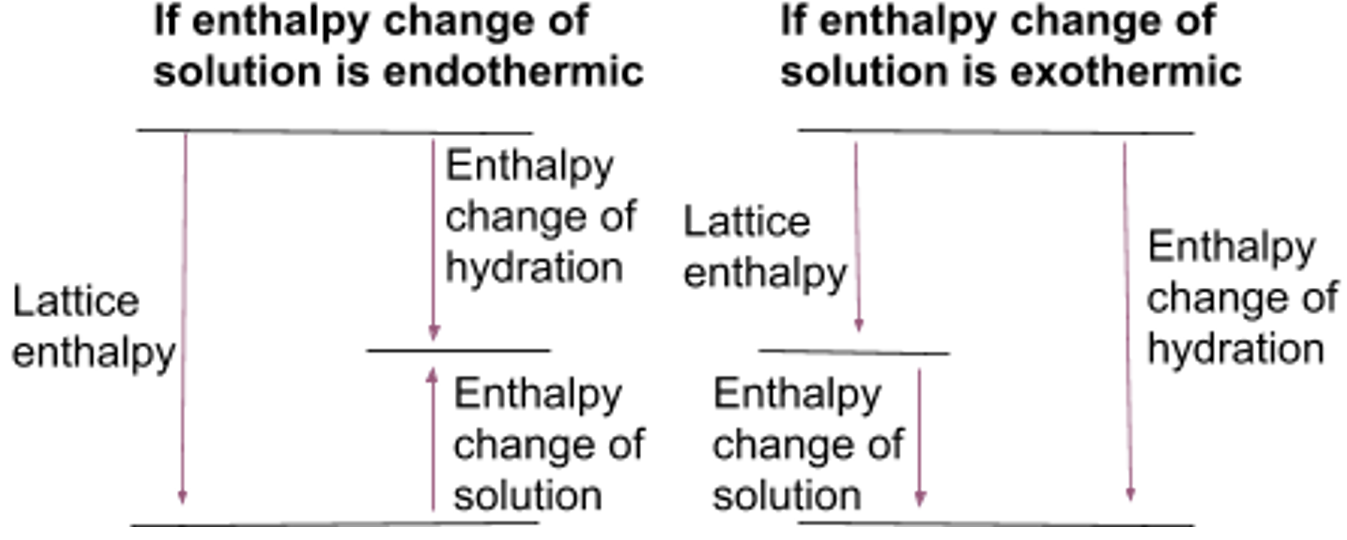
Predicting solubility
- To dissolve an ionic compound in water, attraction between ions in lattice must be overcome
- Energy needed = lattice enthalpy
- Water molecules are attracted to ions, surrounding them and releasing energy
- Energy released = hydration enthalpy
- If sum hydration enthalpies > magnitude lattice enthalpy the overall the reaction is exothermic so the compound should dissolve
- Solubility also depends on other factors + entropy