4.2.3 Organic synthesis
Definitions
| Term | Definition |
|---|---|
| Fractional distillation | The separation of components in a liquid mixture by their different boiling points into fractions with different compositions |
| Drying agent | An anhydrous solid that readily absorbs water from the mixture to become hydrated |
Practical techniques
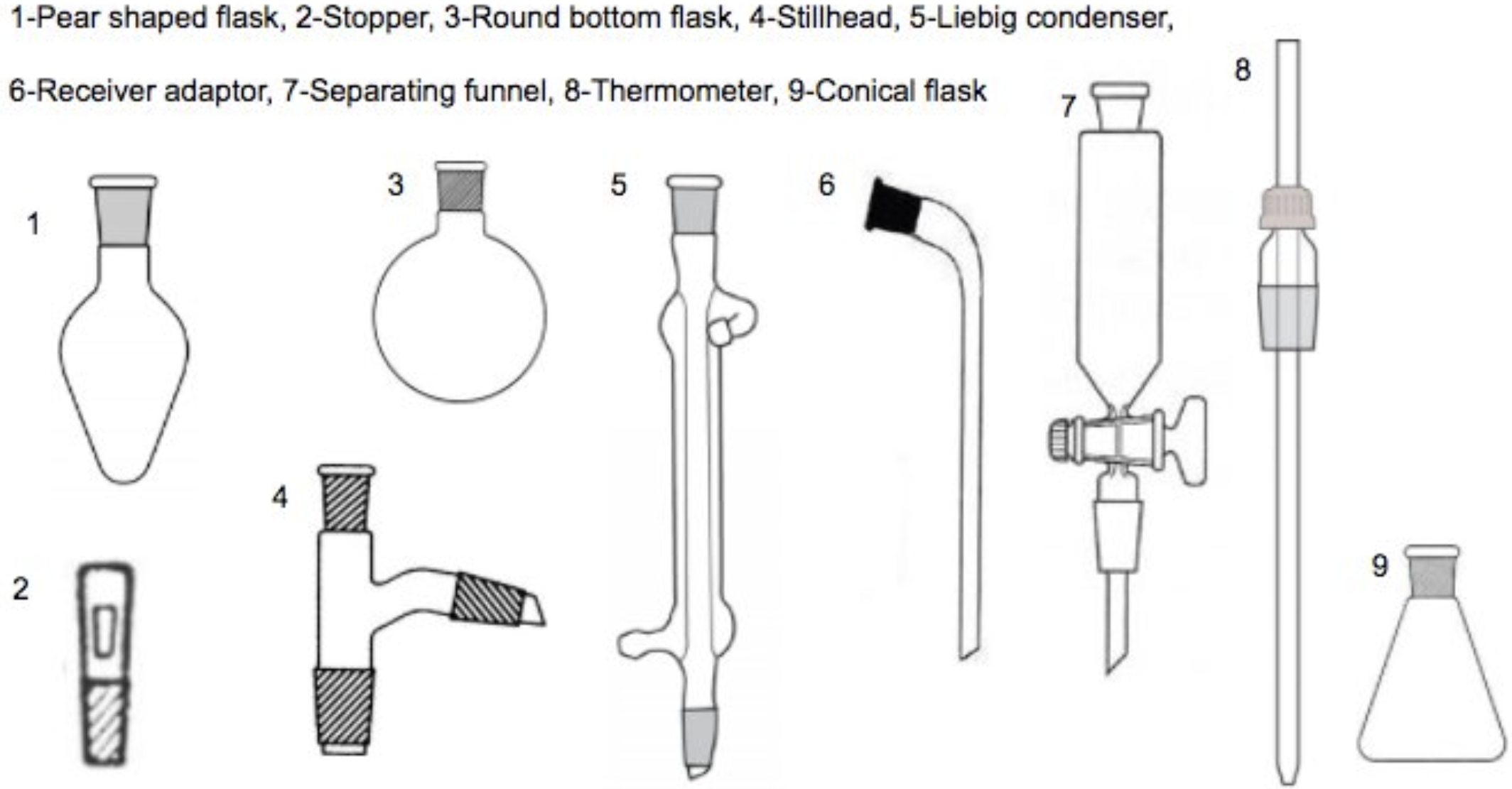
Quickfit apparatus set
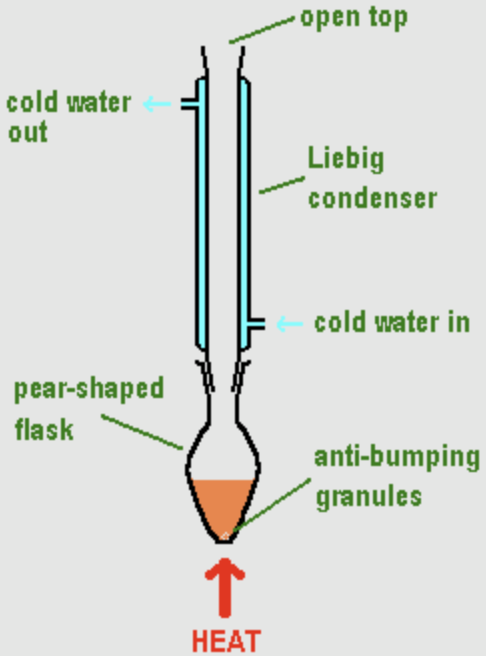
Heating under reflux
- To prepare organic products without boiling off solvent, reactants or products
- Ensures that the reaction goes to completion
- Water bath can be used rather than Bunsen if can be carried out below 100°C
- Heating mantle can be used for flammable liquids
- Anti-bumping granules added to liquid so it boils smoothly
- Otherwise large bubbles will form at bottom so the glassware vibrate / jump
- Glass joints greased lightly so apparatus comes apart easily after experiment
- Condensers should be clamped loosely as the outer jacket is very fragile + kept in upright position
- Never put stopper in top-closed system or pressure would build up and the apparatus would explode
- Rubber tubing used to connect the inlet of condenser to tap and outlet to the sink (water always enters the condenser at the bottom and leaves at the top)
Distillation
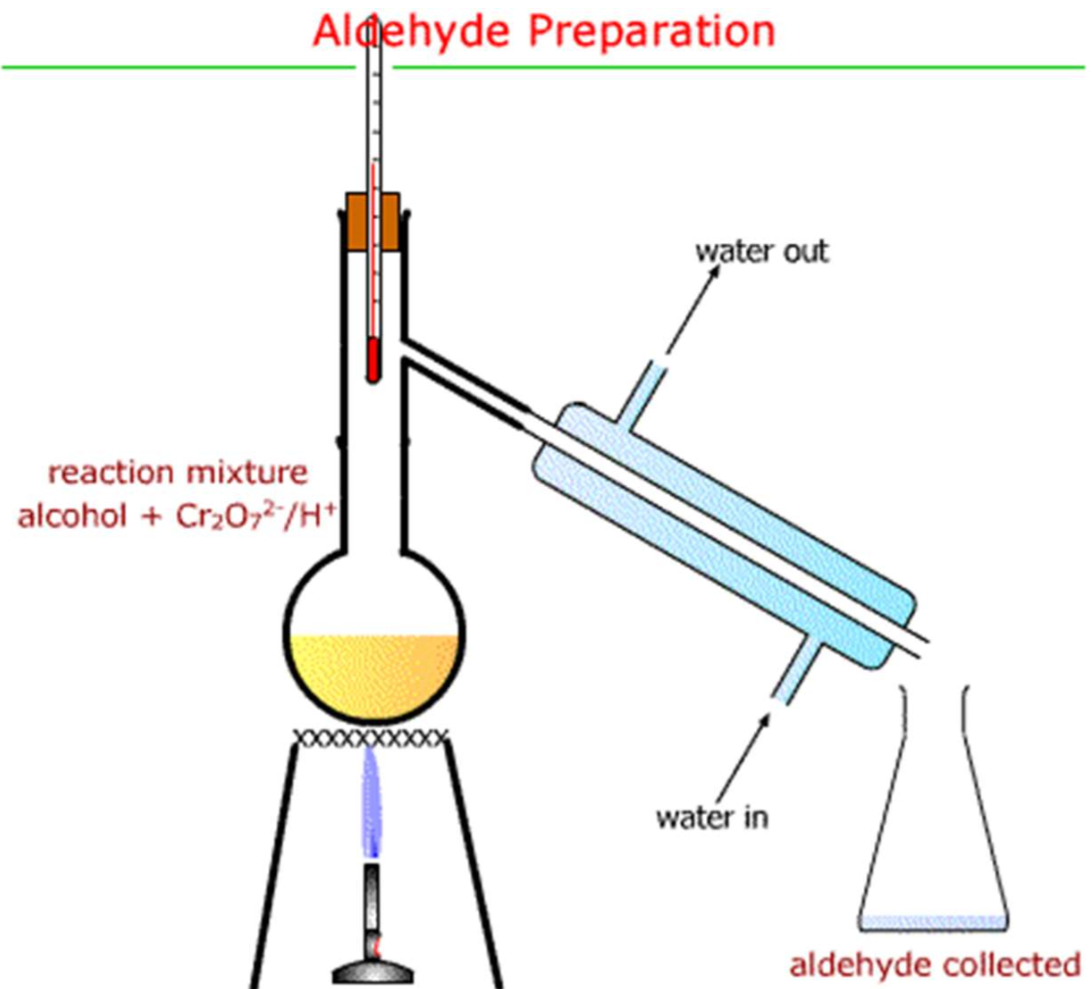
- Separates a pure liquid from impurities
- Flask clamped by neck
- Still head connected to the flask
- Condenser connected to rubber tubing for water (water enters at the bottom)
- Flask used to collect the distillate so the apparatus is not airtight
- Heat the mixture gently (make sure the temperature doesn't reach the boiling point of the less volatile compound)
Purifying organic products using a separating funnel
- When there are two layers inside the collection flask: one organic layer, one aqueous / water layer
- Ensure tap of the separating funnel is closed
- Pour in mixture and place a stopper in the top of the funnel + invert to mix the contents
- Allow layers to settle
- Can't tell the layers: add water to the mixture, the layer that increase in volume is the aqueous layer
- Place conical flask under the separating funnel
- Remove stopper + open the tap until whole lower layer has left the funnel
- If the top layer is accidentally poured then pour the content in the first conical flask back into the separating funnel and restart
- Repeat this several times until the bottom layer is almost completely removed
Redistillation
- Organic compounds may have relatively close boiling points so the sample may still have some impurities left over
- Carry out a second distillation (or more)
- Only collect product with the exact boiling point of the target compound
- Try to not overheat the mixture
- Narrower boiling point range = purer product
Drying an organic product
- There may be water left in the organic product
- Add organic liquid to conical flask
- Add some drying agent with spatula + swirl the contents
- e.g. \(CaCl_2\) for drying hydrocarbons, \(CaSO_4 / MgSO_4\) for general drying
- Put a stopper on to prevent product from evaporating away
- Leave for about 10 minutes
- If the solid stuck in a lump water is still present so add more drying agent until it becomes a fine powder
- After all the water is absorbed the organic mixture can be separated by filtration / simply decanting the liquid
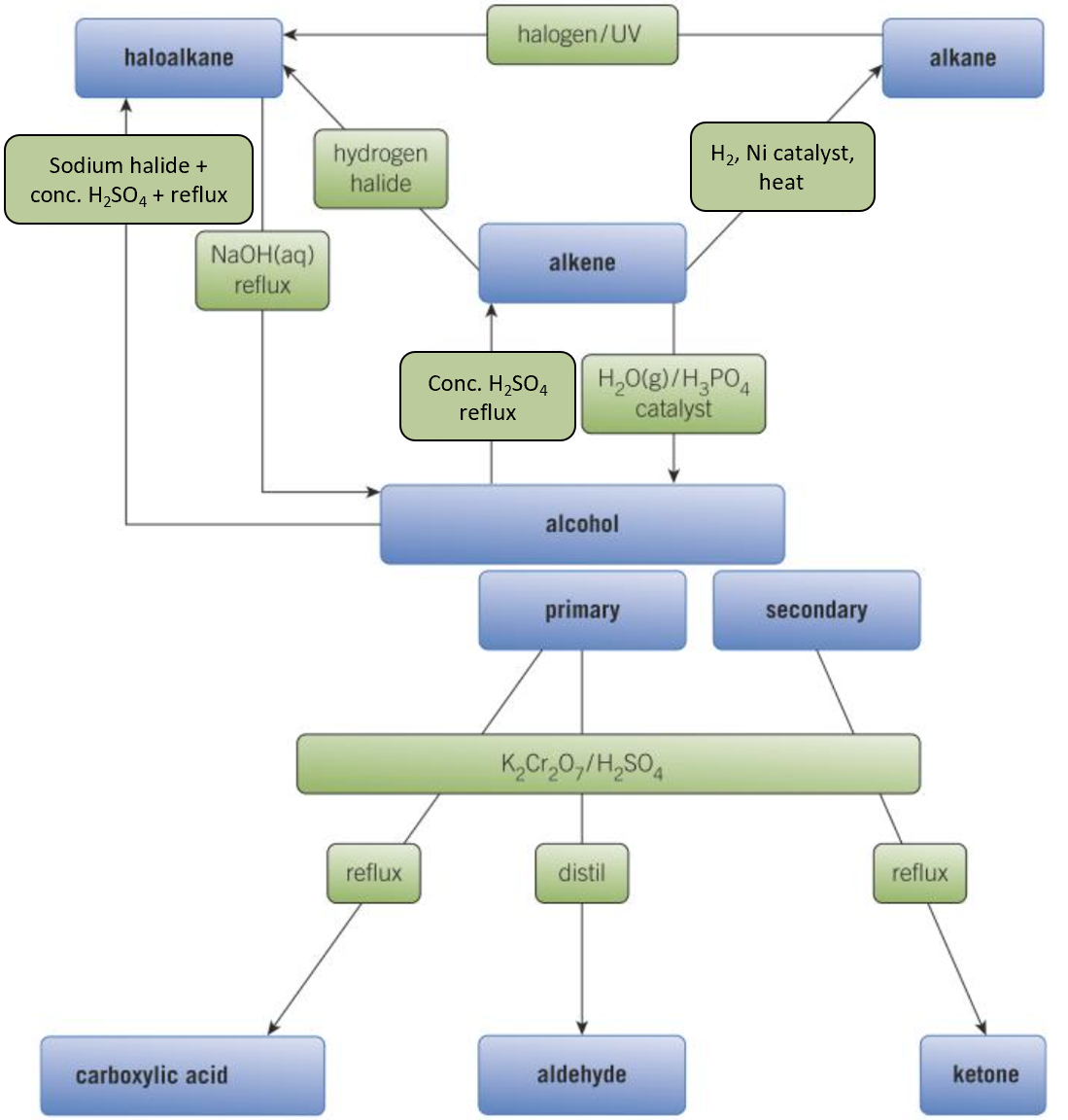
Synthetic routes