4.2.2 Haloalkanes
Definitions
| Term | Definition |
|---|---|
| Nucleophile | An atom or group of atoms which is attracted to an electron-deficient centre or atom, where it donates a pair of electrons to form a new covalent bond |
| Nucleophilic substitution | A reaction in which a nucleophile is attracted to an electron-deficient centre or atom, where it donates a pair of electrons to form a new covalent bond |
| Hydrolysis | A reaction with water that breaks a chemical compound into two compounds, the H and OH in a water molecule becomes incorporated into the two compounds |
Haloalkane reactions
Reactivity of haloalkanes
- Reactivity: alkenes > haloalkanes > alkanes
- Halogen atoms are more electronegative than carbon atoms so the carbon-halogen bond is polar
- \(\delta+\) on carbon can attract nucleophiles (contain a lone pair of electrons)
- The nucleophile replaces the halogen atom
- A new compound with a different functional group is formed
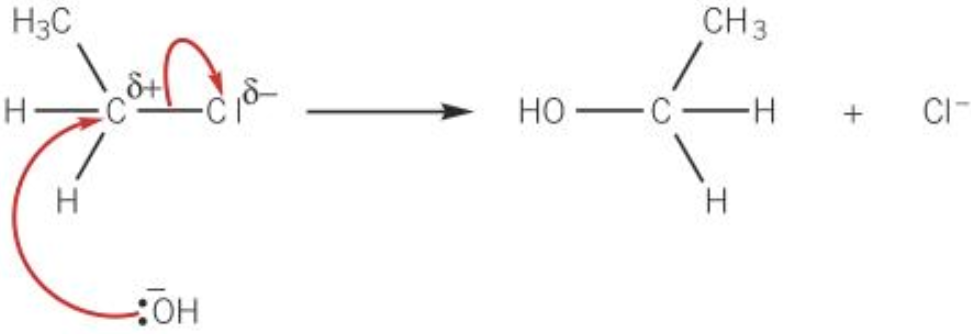
Hydrolysis mechanism
- Nucleophilic substitution
- \(OH^-\) normally from \(NaOH\)
- \(OH^-\) (nucleophile) approaches the carbon atom attached to the halogen from the opposite side of halogen to minimise repulsion
- A lone pair on OH attracted & donated to \(\delta+\) carbon atom
- New bond formed between oxygen atom of OH and the carbon atom
- Carbon-halogen bond breaks by heterolytic fission
- Alcohol + halide ion formed
Trend in reaction rates of hydrolysis of primary haloalkanes
- \(C-F\) has the greatest bond enthalpy (strongest), \(C-I\) has the lowest bond enthalpy (weakest)
- Going down the table = larger halogen atom = longer bond = bond becomes weaker
- Rate: iodoalkanes > bromoalkanes > chloroalkanes > fluoroalkanes
- Rate increases as the bond enthalpy of carbon-halogen bond decreases
- \(C-I\) bond is the weakest (lowest bond enthalpy), \(C-F\) bond is the strongest (highest bond enthalpy)
- Less energy is needed to break the carbon-halogen bond to start the reaction so the activation energy is lower
Measuring rate of hydrolysis
- Set up 3 test tubes of 1 \(cm^3\) ethanol and couple drops of 1-chlorobutane / bromobutane / iodobutane
- Slow down the reaction so we can measure the reaction time easier
- Put the test tubes + a test tube with silver nitrate in water bath at 60°C
- Allow them to reach constant temperature (60°C)
- Add 1 \(cm^3\) of silver nitrate to each test tube quickly + start stop watch
- Observe time taken for precipitate to form
- Chlorine = white, bromine = cream, iodine = yellow
- Speed: iodobutane > bromobutane > chlorobutane
Organohalogen compounds and the ozone layer
CFCs
- Shorthand for chlorofluorocarbons
- Compounds containing carbon with chlorine and fluorine atoms attached
Uses of CFCs
- CFCs are non-flammable and not very toxic so they have a lot of uses
- Refrigerants
- Propellants for aerosols
- Generating foamed plastics
- Solvents for dry cleaning and for general degreasing purposes
Problems associated with CFCs
- Global warming
- Breakdown of ozone layers in the atmosphere
Ozone layer
- Ozone continually formed and broken down by the action of UV radiation
- Initially very high energy UV breaks oxygen molecules into oxygen radicals: \(O_2 \rightarrow 2O\)
- A steady state then set up where rate of ozone formation is the same as the rate of ozone being broken down: \(O_2 + O \rightleftharpoons O_3\)
- Equilibrium disturbed by human activities e.g. production and use of CFCs
How CFCs break down ozone
- CFCs remain stable until they reach the stratosphere
- In the stratosphere UV breaks carbon-halogen bond by homolytic fission to form radicals (initiates the breakdown of ozone)
- Photodissociation (Initiation): e.g. \(CF_2Cl_2 \rightarrow CF_2Cl\bullet + Cl\bullet\)
- Chlorine radical formed is a very reactive intermediate and can react with an ozone molecule
- Propagation step 1: \(Cl\bullet + O_3 \rightarrow ClO\bullet + O_2\)
- Propagation step 2: \(ClO\bullet + O \rightarrow Cl\bullet + O_2\)
- (Overall: \(O_3 + O \rightarrow 2O_2\))
- There is a significant amount of \(O_3\) and free oxygen atoms in the upper atmosphere for reaction
- Chlorine radical can go on in chain reaction to break down other ozone molecules
How nitrogen oxide break down ozone
- Reaction with NO
- Initiation: \(NO \rightarrow N\bullet + O\bullet\)
- Propagation step 1: \(N\bullet + O_3 \rightarrow \bullet NO + O_2\)
- Propagation step 2: \(\bullet NO + O \rightarrow N\bullet + O_2\)
- Overall: \(O_3 + O \rightarrow 2O_2\)
- Reaction with \(NO_2\)
- Initiation: \(NO_2 \rightarrow NO\bullet + O\bullet\)
- Propagation step 1: \(NO\bullet + O_3 \rightarrow NO_2\bullet + O_2\)
- Propagation step 2: \(NO_2\bullet + O \rightarrow NO\bullet + O_2\)
- Overall: \(O_3 + O \rightarrow 2O_2\)
Alternatives for CFCs
- Replace the C-Cl bond with stronger C-F bond
- Hydrochlorofluorocarbons (HCFCs) or hydrofluorocarbons (HFCs) can be used
- Still volatile, non-toxic and non-flammable
- Still damage the ozone layer
- Replace the C-Cl bond with a C-H bond
- Use hydrocarbons
- The C-H bond is much weaker and the molecules don't persist until they reach the upper atmosphere
- They are very flammable