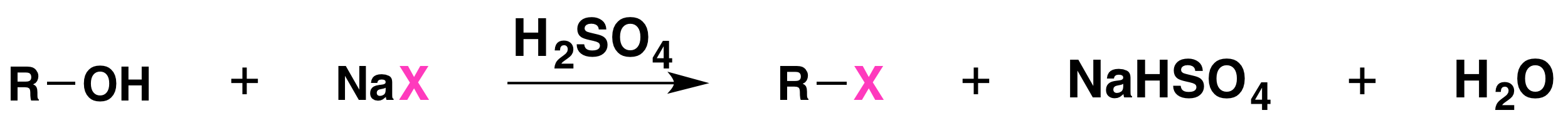4.2.1 Alcohols
Definitions
| Term | Definition |
|---|---|
| Dehydration | A water molecule is removed from the starting material |
Properties of alcohols
Alcohol structure
- Functional group = \(-OH\) (hydroxyl group)
- Has polar and non-polar parts
- The \(O-H\) bond is polar (oxygen is more electronegative than hydrogen)
- The side chain is non-polar
- so alcohol can mix with both polar and non-polar liquids
Alcohol properties
- Higher melting & boiling point than alkanes
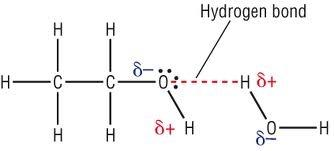
- Induced dipole-dipole interactions between the non-polar side chains
- Strong hydrogen bonds + permanent dipole-dipole interactions between alcohol molecules hold them together (stronger than London forces)
- Extra heat energy is required to break the strong hydrogen bonds
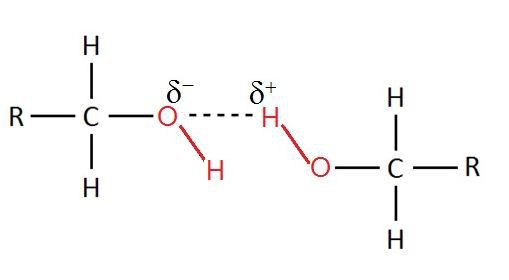
- Greater water solubility than alkenes
- Alkanes are non-polar so they cannot form hydrogen bonds / PDD with water
- \(O-H\) bond in alcohol is polar and forms hydrogen bond with water
- Longer carbon chain = less miscible in water
- More \(-OH\) groups = more miscible in water
Classifying alcohols
- Primary: \(-OH\) group attached to a carbon atom attached to 2 hydrogen atoms + 1 alkyl group
- Secondary: \(-OH\) group attached to a carbon atom attached to 1 hydrogen atom + 2 alkyl groups
- Tertiary: \(-OH\) group attached to a carbon atom attached to no hydrogen atoms + 3 alkyl groups
Reactions of alcohols
(Complete) combustion of alcohols
- Alcohol + oxygen \(\rightarrow\) carbon dioxide + water
- Exothermic reaction
- A large quantity of energy released in the form of heat
- Burn with a clear blue flame
- More carbon atoms in the alcohol chain = more heat energy released per mole
- Alcohols undergo complete combustion more often than alkanes due to the oxygen atom in the molecule
Oxidation of alcohols
- Heat with an oxidising agent ([O])
- Normally acidified dichromate (VI) (\(Cr_2O_7^{2-} / H^+\))
- e.g. acidified potassium dichromate (VI) (\(K_2Cr_2O_7 / H_2SO_4\))
- Observations
- Cr will be reduced
- Reaction mixture turn from orange (\(Cr^{6+}\) in \(Cr_2O_7^{2-}\)) to green (\(Cr^{3+}\))
Oxidation of primary alcohols
- Gentle heating + distillation = aldehyde formed
- Aldehyde distilled out of the reaction mixture as it forms to prevent any further reaction (distil)
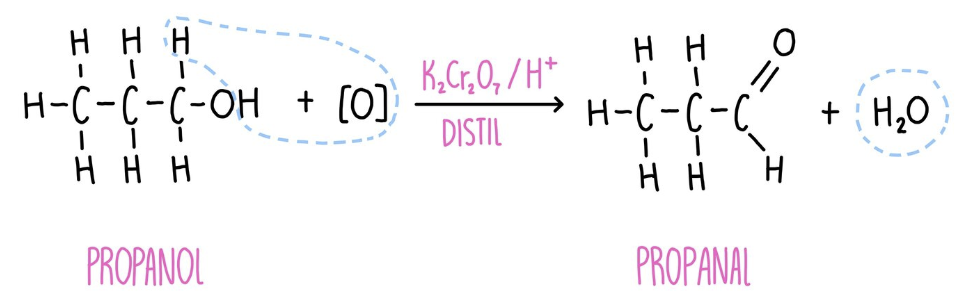
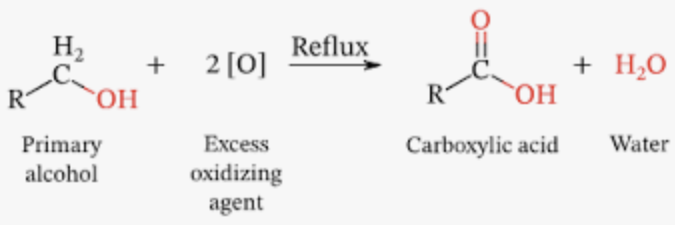
- Heated strongly + reflux + excess of acidified potassium dichromate (VI) = carboxylic acid
- Reflux = ensure that all aldehyde formed initially also oxidised to carboxylic acid
- Excess of oxidising agent = ensure that all alcohol is oxidised
Oxidation of secondary alcohols
- Oxidised to ketones with an oxidising agent (acidified dichromate (VI) ions)
- Ketones cannot be further oxidised
- Heated under reflux with the oxidising agent to ensure that the reaction goes to completion
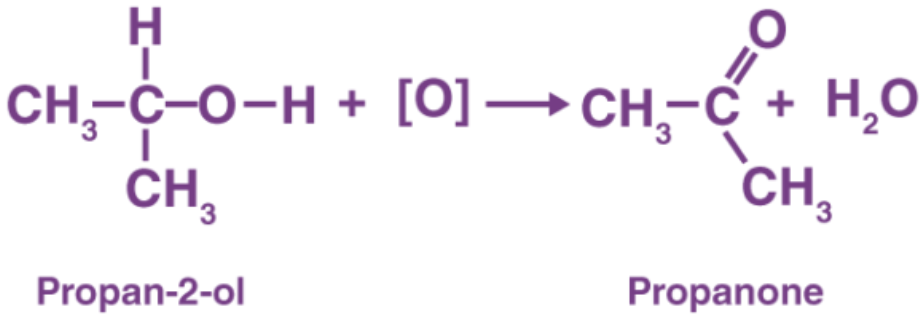
Oxidation of tertiary alcohols
- Do not undergo oxidation reactions
- Acidified dichromate (VI) ions remain orange when added to a tertiary alcohol
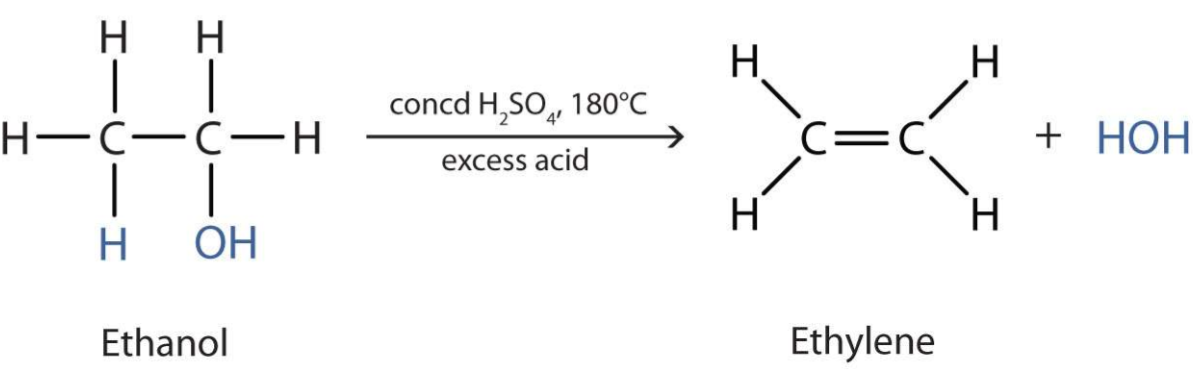
Dehydration of alcohols
- Heated under reflux with concentrated acid catalyst (e.g. concentrated \(H_2SO_4 / H_3PO_4\))
- Product = an alkene
- Type = elimination reaction
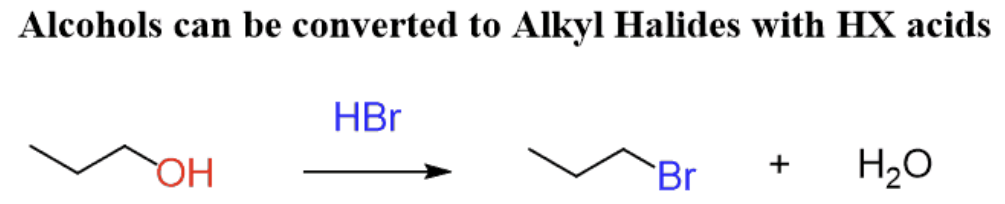
Substitution reaction of alcohols
- Alcohols react with halide ions to form haloalkanes
- Heated under reflux with concentrated acid catalyst & halide ion e.g. \(H_2SO_4 + NaBr\)
- Acid need to be concentrated to minimise back reactions