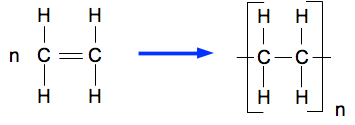
4.1.3 Alkenes
Definitions
| Term | Definition |
|---|---|
| Electrophile | An atom or group of atoms which is attracted to an electron-rich centre of atom, where it accepts a pair of electrons to form a new covalent bond, usually a cation or an atom or molecule with \(\delta+\) dipole |
| Electrophilic addition | An addition reaction in which the first step is attack by an electrophile on a region of high electron density |
| Addition polymerisation | Formation of a very long molecular chain, by repeated addition reactions of many unsaturated alkene molecules (monomers) |
Properties of alkene
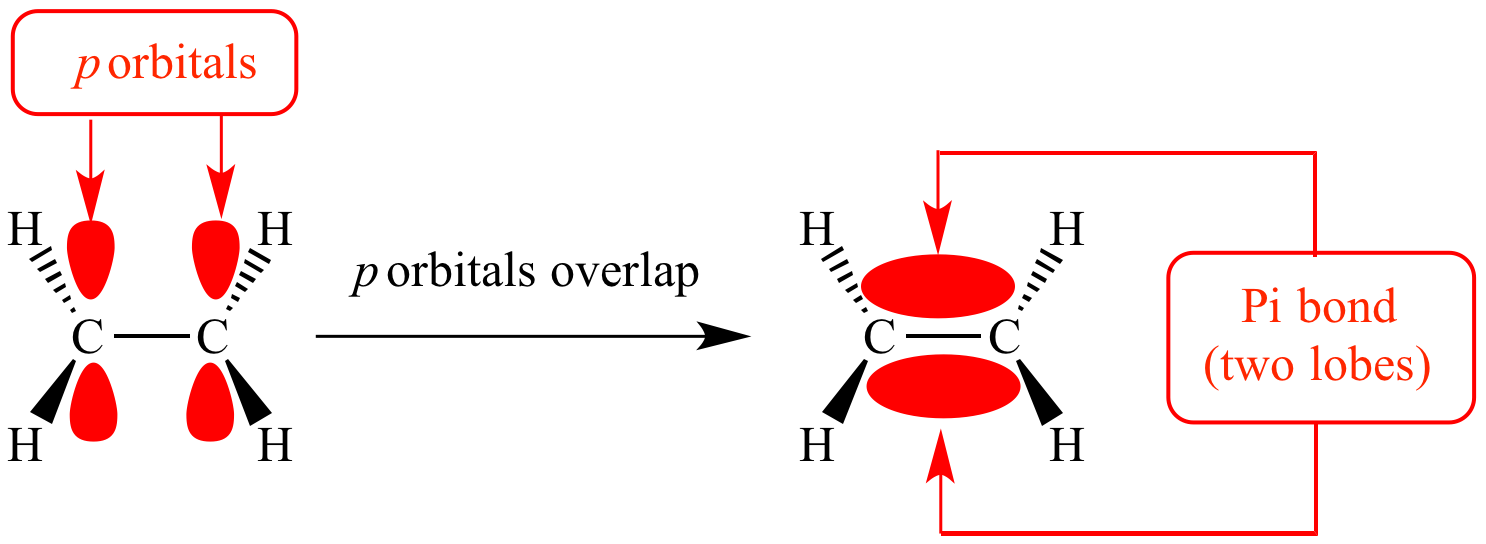
Structure of C=C bond
- Comprised of
- A \(\sigma\)-bond: head on overlap of orbitals directly between the bonding atoms
- A \(\pi\)-bond: sideways overlap of adjacent p-orbitals above and below the bonding carbon atoms
- The \(\pi\)-bond locks the two carbon atoms in position and prevents them from rotating around the double bond (restrict rotation)
- Trigonal planar shape around each carbon atom in the \(C=C\) bond (120° bond angle)
- 3 regions of electron density around each carbon atom (3 bonding regions)
- The 3 regions repel each other as far apart as possible
\(\sigma\) and \(\pi\)-bond difference
| \(\sigma\)-bond | \(\pi\)-bond | |
|---|---|---|
| Position of electron density | Between bonding atoms | Above and below bonding atoms |
| Overlap of orbitals | Head on overlap of orbitals | Sideways overlap of orbitals |
| Bond enthalpy / strength | Higher | Lower |
| Size | Larger | Smaller |
Stereoisomerism in alkenes
Stereoisomer
- Compounds with the same structural formula but with a different arrangement in space
E/Z isomerism / geometrical isomerism
- An type of stereoisomerism
- Different groups attached to each carbon atom of a \(C=C\) double bond may be arranged differently in space because of the restricted rotation about the \(C=C\) bond
- Rotation about a double bond is restricted (due to the \(\pi\)-bond) so the groups attached to each carbon atom are fixed relative to each other
Conditions for E/Z isomerism
- A \(C=C\) double bond
- Two different groups to be attached to each carbon atom of the double bond
Cis–trans isomerism
- A special case of E/Z isomerism
- One of the attached groups on each carbon atom of the double bond must be the same
- Same group on same side = cis, same group on different sides = trans (only works if there is a hydrogen atom bonded to both carbon atoms)
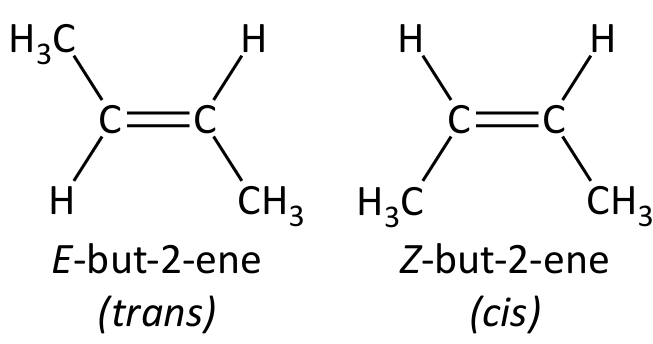
Identify E/Z isomers by Cahn-Ingold-Prelog (CIP) priority rules
- Assigning priority
- Examine the atomic number of the atoms directly attached to the carbon atoms of the double bond
- Higher atomic number = higher priority
- Two same atoms attached to the carbon atom
- Find the first point of difference
- Higher atomic number at first point of difference = higher priority
- The groups of higher priority are on the same side = Z isomer
- The groups of higher priority are diagonally placed across the double bond = E isomer
Addition reactions of alkenes
Reactivity of alkenes
- Much more reactive than alkanes
- Relative low bond enthalpy of the \(\pi\)-bond so it is broken more readily
- It is on the outside of the \(\sigma\)-bond so its electrons are more exposed
Addition reactions of alkanes
| Reaction | Condition | Detail |
|---|---|---|
| Hydrogenation | - Nickel catalyst - 423 K (150°C) - High pressure |
- Alkene + hydrogen \(\rightarrow\) alkene / \(R-CH=CH_2 + H_2 \rightarrow R-CH_2-CH_3\) - Type: hydrogenation / addition |
| Halogenation | - RTP | - Alkene + halogen \(\rightarrow\) dihaloalkane e.g. \(R-CH=CH_2 + Br_2 \rightarrow R-CHBr-CH_2Br\) - Type: electrophilic addition (see below for mechanism) - Reaction of alkenes with bromine can be used to test if the organic compound is unsaturated - Bromine water added dropwise to alkene - Bromine adds across the double bond - The orange colour of bromine water disappears - Added to an saturated compound: no addition reaction so no colour change |
| Addition with (gaseous) halogen halides | - RTP | - Alkene + halogen halide \(\rightarrow\) haloalkane e.g. \(R-CH=CH_2 + HBr \rightarrow R-CHBr-CH_3\) - Type: electrophilic addition (see below for mechanism) - Alkene is a gas: reaction takes place when the two gases are mixed - Alkene is a liquid: hydrogen halide bubbled through it - Can also react with concentrated hydrochloric or hydrobromic acid - Two possible products |
| Hydration | - Steam - Phosphoric acid (\(H_3PO_4\)) catalyst |
- Alkene + \(H_2O(g) \rightarrow\) alcohol - Type: hydration - \(R-CH=CH_2 + H_2O \rightarrow R-CH(OH)-CH_3\) - Two possible products |
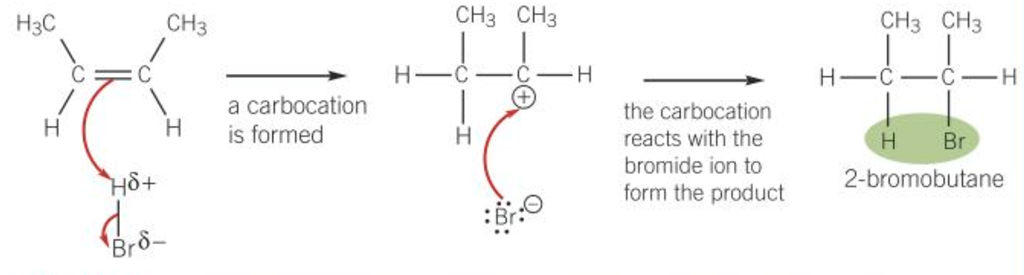
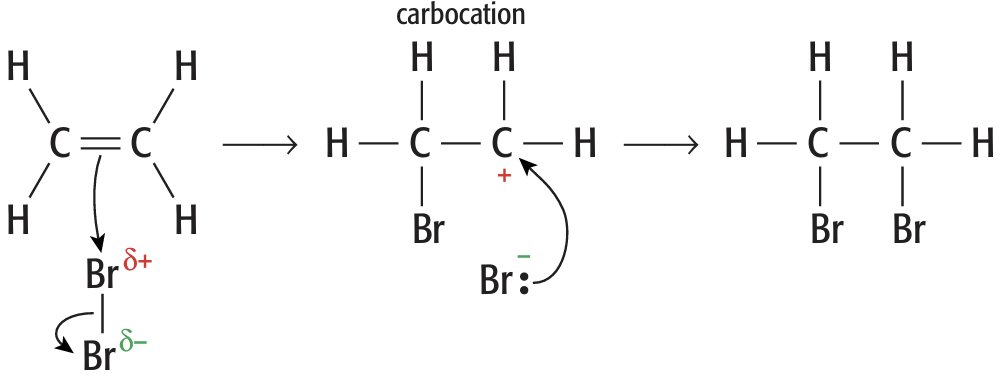
Electrophilic addition mechanisms
- Electrophile = \(\delta+\) atom (accepts the \(\pi\)-electrons from the double bond)
- Electron pair in the \(\pi\)-bond is attracted to the \(\delta+\) atom \(\rightarrow\) double bond breaks
- A bond forms between the \(\delta+\) atom and a carbon atom from the double bond
- The bond in the molecule breaks by heterolytic fission, electron pair goes to the \(\delta-\) atom
- An anion and a carbocation (positively charged carbon atom) are formed
- They react to form the addition product
Types of carbocations
| Type | Definition |
|---|---|
| Primary | 1 alkyl group attached to the positively charged carbon atom |
| Secondary | 2 alkyl groups attached to the positively charged carbon atom |
| Tertiary | 3 alkyl groups attached to the positively charged carbon atom |
Using Markownikoff's rule to predict formation of major organic product
- For unsymmetrical alkanes
- Major product is formed from the most stable carbocation intermediate
- Stability: tertiary carbocation > secondary carbocation > primary carbocation
- Halide / \(OH^-\) ion attached to the carbon atom attached to the carbon atom with the least hydrogens attached / most alkyl groups attached
- The hydrogen attaches itself to the carbon atom with the most hydrogens attached
Polymers
Addition polymerisation of alkenes
- Short chain monomers join together to form long chain polymers under high pressure
- Double bond of the alkene is replaced by single bonds to form a repeating unit + bond with other monomers to form the polymer
- Addition polymers as the short chains join together to form a single product
Problems of waste polymers
- Benefits of cheap oil-derived plastics are counteracted by problems for the environment of landfill
- They are unreactive so they are non-biodegradable and cannot be broken down by species in nature
- Non-biodegradable waste polymers can become a threat to wildlife
Reducing the effect of waste polymers
- Choose plastic items that are made from polymers that can be recycled
- Re-use plastic items at many time as possible
- Try to recycle plastic items
Ways of processing waste polymers
- Recycle
- High cost of collection and re-processing
- The different types of polymer have to be separated
- Combustion to release heat energy for generating electricity
- Toxic fumes produced from burning halogenated polymers
- HCl is removed during the combustion of chlorine containing haloalkanes
- CO produced during incomplete combustion
- Can be removed by scrubbing in the chimney
- Greenhouse gases can be released which causes global warming
- Toxic fumes produced from burning halogenated polymers
- Organic feedstock
- Use the waste for the production of useful organic compounds
- New technology can convert waste into hydrocarbons
- Hydrocarbons can then be turned back into polymers
New types of polymers
- Biodegradable polymers
- Broken down by microorganisms into water, \(CO_2\) and organic compounds
- Compostable polymer degrade and leave no visible or toxic residues
- e.g. can be used as bin liners for food waste
- Photodegradable polymers
- Contain weak bonds that break when they absorb light energy
- Benefits
- Conserve fossil fuel reserves
- Reduce pollution from disposing polymers