4.1.2 Alkanes
Definitions
| Term | Definition |
|---|---|
| Free radical | A species with an unpaired electron |
| Chain reaction | A reaction in which the propagation steps release new radicals that continue the reaction |
| Initiation | The first stage in a radical reaction in which radicals form when a covalent bond is broken by homolytic fission |
| Propagation | The steps that continue a free radical reaction, in which a radical reacts with a reactant molecule to form a new molecule and another radical, causing a chain reaction |
| Termination | The step at the end of a radical substitution when two radicals combine to form a molecule |
Properties of alkanes
Bonding in alkanes
- Saturated hydrocarbons
- Only carbon and hydrogen atoms joined together by single covalent bonds
- Bond type = \(\sigma\)-bond (sigma bond)
- \(\sigma\)-bond = heads on overlap of orbitals directly between the bonding atoms
- One orbital from each bonding atom, each containing one electron
- Positioned on a line directly between bonding atoms

- Atoms can rotate freely around the \(\sigma\)-bond
Shape of alkanes
- Tetrahedral shape around each carbon atom, bond angle 109.5°
- Each carbon atom surrounded by 4 bonded pairs in four \(\sigma\)-bonds
- The electron pairs repel each other as far away as possible
Effect of chain length on boiling points
- Longer chain length = higher boiling point
- Increased chain length = greater surface area of contact + more electrons
- Stronger London forces
- More energy is required to overcome the London forces
Effect of branching on boiling points
- More branching = lower boiling point
- More branches = fewer surface area of contact \(\rightarrow\) weaker London forces
- The branches prevent the branched molecules getting as close together as straight-chain molecules \(\rightarrow\) further decrease intermolecular forces
- Less energy is required to overcome the London forces
Alkane reactions
Reactivity of alkanes
- Low reactivity
- \(C-C\) and \(C-H\) \(\sigma\)-bonds are strong as they have a high bond enthalpy
- \(C-C\) bonds are non-polar
- \(C-H\) bond can be considered non-polar as the electronegativities of C and H are very similar
Combustion of alkanes
- Complete combustion (sufficient oxygen present)
- Equation: \(C_{x} H_{2 x + 2} + (\frac{3 x + 1}{2}) O_{2} \rightarrow x CO_{2} + (x + 1) H_{2} O\)
- Incomplete combustion (insufficient oxygen present)
- Hydrogen atom always oxidised to water
- Combustion of carbon may be incomplete so carbon (soot) or carbon monoxide is formed instead of \(CO_2\)
- Carbon monoxide is toxic + colorless and odorless so it is difficult to spot
- CO combines irreversibly to haemoglobin and replace oxygen so oxygen cannot pass round the body and the person can suffocate
- Alkane is a good source of fuel
- Release large amounts of energy when burned
- Easy to transport
Methane and chlorine reaction
- Equation: \(R-CH_3 + X_2 \rightarrow R-CH_2X + HX\)
- Type: free radical substitution
- Step 1: initiation
- The halogen-halogen bond is broken by homolytic fission to form 2 free radicals
- Energy for bond fission is provided by UV radiation
- e.g. \(Cl_2 \xrightarrow{u.v.} 2Cl\bullet\)
- Step 2: propagation (halogen radical intermediate react with original reactants)
- One free radical reacts to produce another different free radical
- Always in 2 steps
- First propagation step: \(Cl\bullet + CH_4 \rightarrow \bullet CH_3 + HCl\)
- Second propagation step: \(\bullet CH_3 + Cl_2 \rightarrow CH_3Cl + Cl\bullet\)
- A halogen radical acts as an catalyst and is recreated
- Step 3: termination
- Two free radicals combine and their unpaired electrons pair up to form a covalent bond between the 2 species
- \(Cl\bullet + \bullet Cl \rightarrow Cl_2\) / \(\bullet CH_3 + \bullet CH_3 \rightarrow C_2H_6\) / \(\bullet CH_3 + \bullet Cl \rightarrow CH_3Cl\)
- Both radicals are removed from the reaction mixture so the reaction stops
- (Same equation for bromine atoms)
Skeletal formulae for free radical substitution
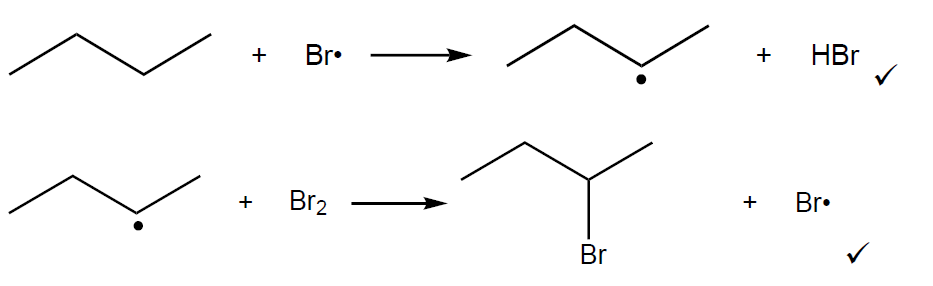
- Dot on the carbon atom with hydrogen atom removed
Limitations of radical substitution in synthesis
- Further substitution
- The propagation step can continue many times as it is a chain reaction
- Conditions can be altered to favour the termination step and limit the number of substitutions
- Substitution at different positions in a carbon chain
- Longer chain = a mixture of monosubstituted isomers formed by substitution at different positions of the chain
- Different chains can also undergo further substitution
- Produces different termination products (more than one possible termination step)