3.2.2 Reaction rates
Definitions
| Term | Definition |
|---|---|
| Intermediate | A species formed during a reaction that reacts further and is not present in the final products |
Rate of reaction
Rate of reaction
- Measures how fast a reactant is being used up / a product is being formed
- \(\text{rate} = \frac{\text{change in concentration}}{\text{time}}\) (units = \(mol \cdot dm^{-3} \cdot s^{-1}\))
Measuring rates of reaction by mass loss
- Add reactants to conical flask on a digital mass balance + start stopwatch timer
- Mass is recorded initially & at regular intervals (state a value e.g. 10s) until no more mass lost
- Plot a graph of mass against time
- Gradient of tangent at t = 0 is the initial rate
The collision theory
The collision theory
- Two reacting particles must collide with the correct orientation and have sufficient energy to overcome the activation energy barrier of the reaction to react
- Most collisions between particles are unsuccessful and do not result in a chemical reaction
Effect of change in concentration / pressure on rate of reaction
- Increase concentration / pressure = increase in rate of reaction
- Increases the number of particles in the same volume
- The particles are closer together and collide more frequently
- In a given period of time there will be more effective collisions
- (The proportion of collisions that are successful does not change)
Catalyst
Catalyst
- Increases the rate of reaction without being used up by the overall reaction
- Allowing a reaction to proceed via a different route with lower activation energy
- May react with a reactant to form an intermediate or provide a surface on which the reaction can take place
Types of catalysts
- Homogenous: The catalyst is in the same physical state as the reactants
- Heterogeneous: The catalyst is in a different physical state from the reactants
Benefits of catalysts
- Obtain industrial products faster
- Increase profit
- Operate industrial processes at lower temperatures and pressures
- Reduce energy demand as less electricity and fuel is used
- Reduce \(CO_2\) emissions due to burning fossil fuels
- Different reactions can be used
- Choose reactions with greater atom economy / less toxic solvents / less toxic reactants
Problems of catalysts
- Catalysts do not last forever and need to be replaced periodically
- Waste need to be disposed of responsibly
- Many catalysts are toxic and need to be disposed of very carefully to prevent damage to the environment
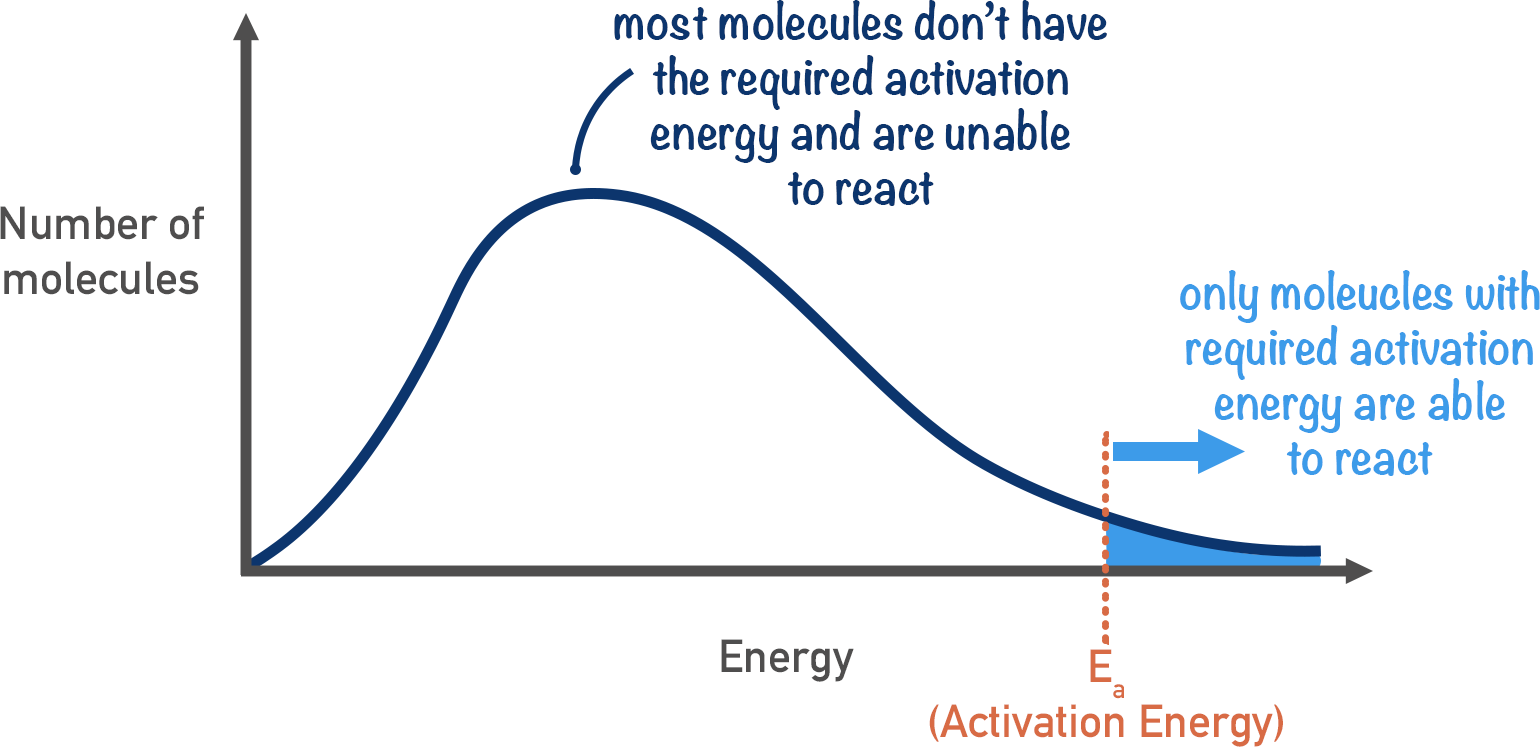
Boltzmann distribution
Boltzmann distribution
- Not all molecules of a substance have the same amount of energy
- Only particles to the right of the \(E_{a}\) have enough energy to react
- Distribution graph
- Area under curve = total number of molecules
- Peak = most probable energy of a molecule
- No molecules have zero energy (graph starts at origin)
- No maximum energy for a molecule (the curve does not meet the x-axis at high energy)
- The shape is positively skewed
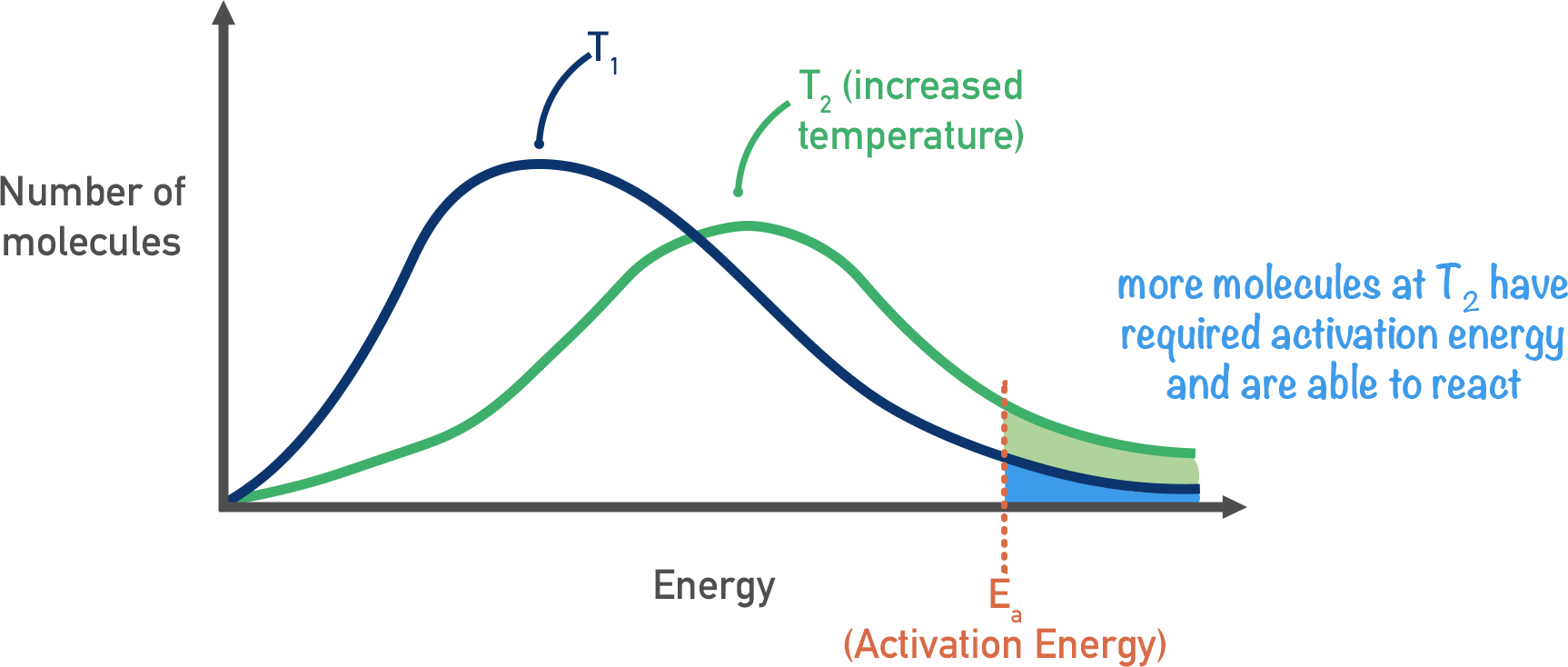
Effect of change in temperature on Boltzmann distribution
- Graph: higher temperature = lower peak, peak shifted to the right
- Larger area to the right of \(E_{a}\) / more molecules have energy \(\ge E_{a}\)
- A greater proportion of collisions will lead to a reaction (major effect)
- More frequent collisions as the molecules gain more KE and are moving faster (minor effect)
- Rate of reaction increases
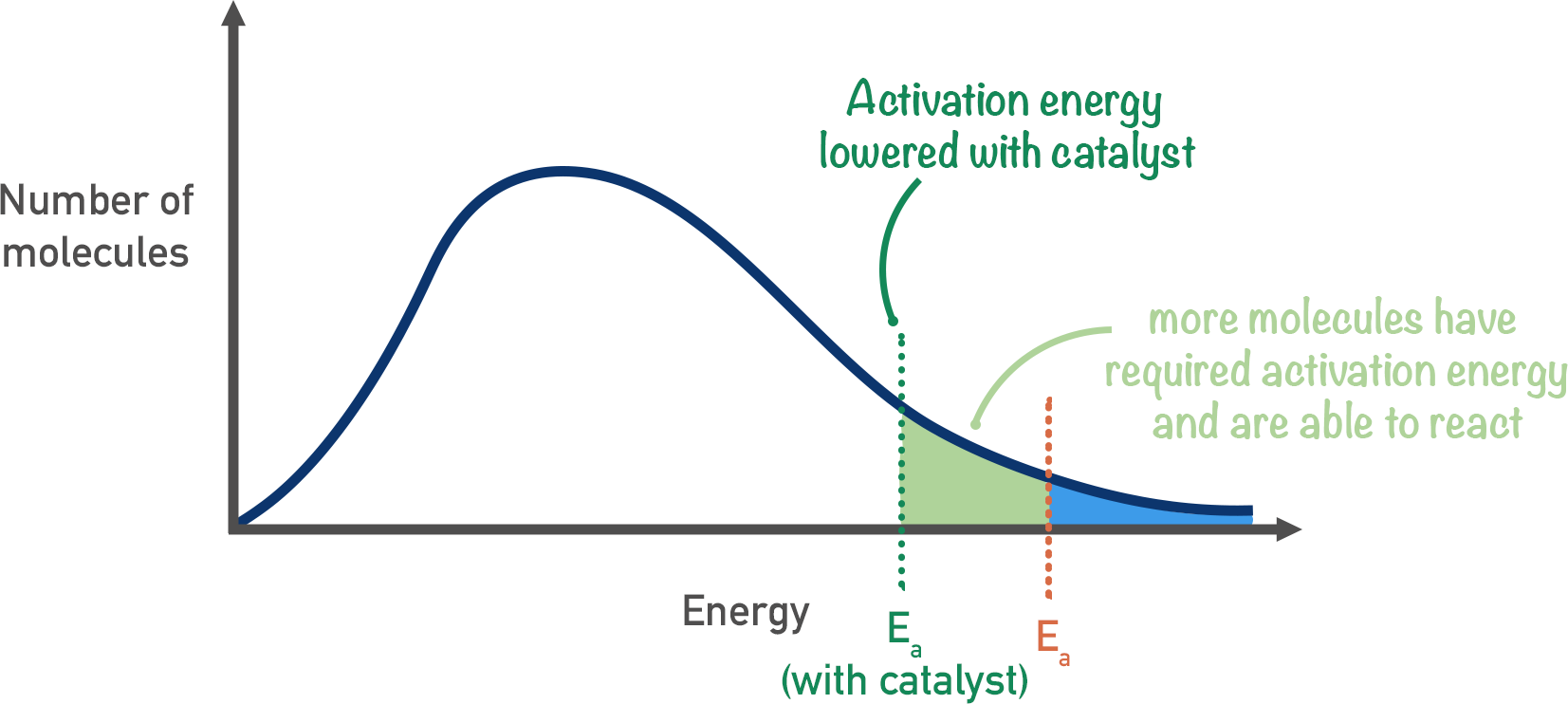
Effect of catalyst on Boltzmann distribution
- Larger area to the right of \(E_{a}\) / more molecules have energy greater than \(E_{a}\)
- A greater proportion of molecules now have an energy \(\ge\) the new lower activation energy
- A greater proportion of collisions will lead to a reaction
- Increases the rate of reaction