3.2.1 Enthalpy changes
Definitions
| Term | Definition |
|---|---|
| Enthalpy | Measure of heat energy in a chemical system |
| Chemical system | The atoms, molecules or ions making up the chemicals |
| Activation energy / \(E_{a}\) | The minimum energy required for a reaction to take place |
| Standard state | The physical state of a substance under standard conditions |
| Enthalpy change of reaction / \(\Delta_{r} H\) | The enthalpy change associated with a stated equation in the molar quantities shown in the equation |
| Enthalpy change of formation / \(\Delta_{f} H\) | The enthalpy change that takes place when 1 mole of a compound is formed from its elements |
| Enthalpy change of combustion / \(\Delta_{c} H\) | The enthalpy change that takes place when 1 mole of a substance reacts completely with oxygen |
| Enthalpy change of neutralisation / \(\Delta_{neut} H\) | The enthalpy change that accompanies the reaction of an acid by a base to form 1 mole of \(H_2O(l)\) |
| Enthalpy change of solution / dissolution | The enthalpy change when 1 mole of a substance is completely dissolved in water |
| Hess's Law | The enthalpy change in a chemical reaction is independent of the route it takes |
(standard ... = under standard conditions : 298 K & 100 kPa, with all reactants and products in their standard states)
Enthalpy changes
Enthalpy change
- \(\Delta H = H(products) - H(reactants)\)
- Can be positive (endothermic) or negative (exothermic)
Types of reactions
- Exothermic reaction: Energy transferred from the system to the surroundings
- Endothermic reaction: Energy transferred from the surroundings to the system
Enthalpy profile diagrams
| Exothermic | Endothermic |
|---|---|
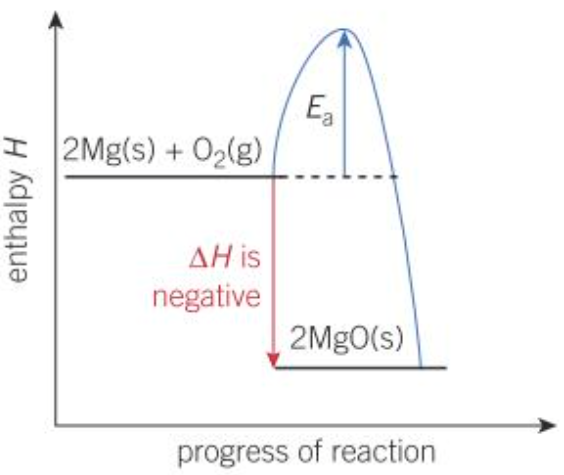 \(\Delta H\) is negative |
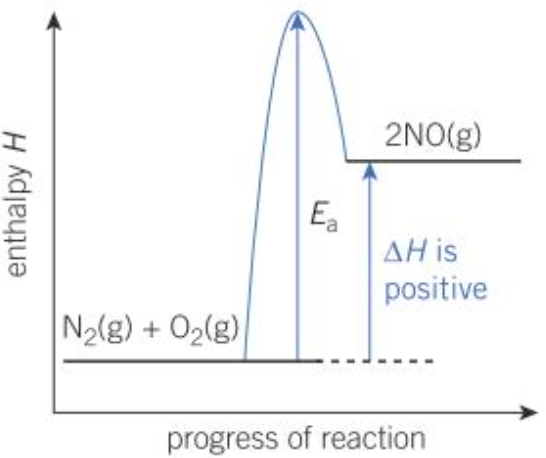 \(\Delta H\) is positive |
- The activation energy can normally be omitted when there are > 1 energy changes
Standard conditions
- Shown with symbol \(^\ominus\) / °
- Standard pressure = 100 kPa / \(1 \times 10^5\) N \(m^{-2}\) / 1 atm
- Standard temperature = 298 K / 25 °C
- Standard concentration = 1 mol \(dm^{-3}\) / 1 M
- Substances will be in their normal physical states (standard states)
Measuring energy changes
- Use the equation \(q = mc\Delta T\)
- Energy change for the system = \(-q\) (if heat lost to surroundings)
Determine enthalpy change of combustion
- Measure certain volume of water, pour water into beaker
- Record initial temperature of water using a thermometer
- Add fuel to spirit burner + weigh spirit burner and fuel together
- Place spirit burner under beaker + light the burner
- Stir water with thermometer
- Extinguish flame after about 3 mins
- Record the maximum temperature reached
- Re-weigh spirit burner
- Work out the mass of fuel burnt hence the amount of fuel burnt in moles
- Work out energy change with \(q = mc\Delta T\)
- \(\text{Enthalpy change of combustion} = -\frac{q}{n}\)
Reasons for inaccuracies
- Heat loss to surroundings \(\rightarrow\) less exothermic than expected
- Incomplete combustion of fuel (black layer soot on calorimeter) \(\rightarrow\) less exothermic than expected
- Non-standard conditions \(\rightarrow\) more or less exothermic than expected
- Evaporation of fuel
- Less exothermic
- Seems to have used more fuel than actual
- Extinguish the spirit burner ASAP after the experiment + reweigh the spirit burner
Determine enthalpy change of reaction
- Carry out reaction in polystyrene cup with thermometer clamped so that it stands in it
- Surroundings = the reaction solution (may assume density and specific heat capacity are the same as water)
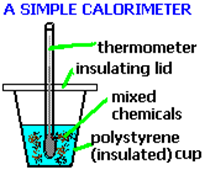
Determining the enthalpy change of neutralisation
- Make one reactant excess so the amount of water formed is fixed
- In theory all acid and alkali should release the same amount of energy but in reality it isn't due to the different strengths of acid / alkali species
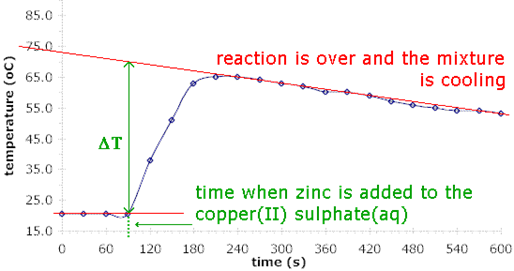
Cooling curve correction
- Add one reactant to the polystyrene cup
- Take temperature of the solution every 30s until temperature stays constant
- Add the other reactant + stir
- Measure temperature every 30s until temperature has fallen for several mins
- Plot a graph of temperature against time
- Extrapolate cooling curve section back to when second reactant added
- Find the theoretical \(\Delta T\) at that point of time
Average bond enthalpy
- The enthalpy change when 1 mole of a bond is broken in the gas state
- Limitations: actual bond enthalpy can vary depending on the chemical environment of the bond
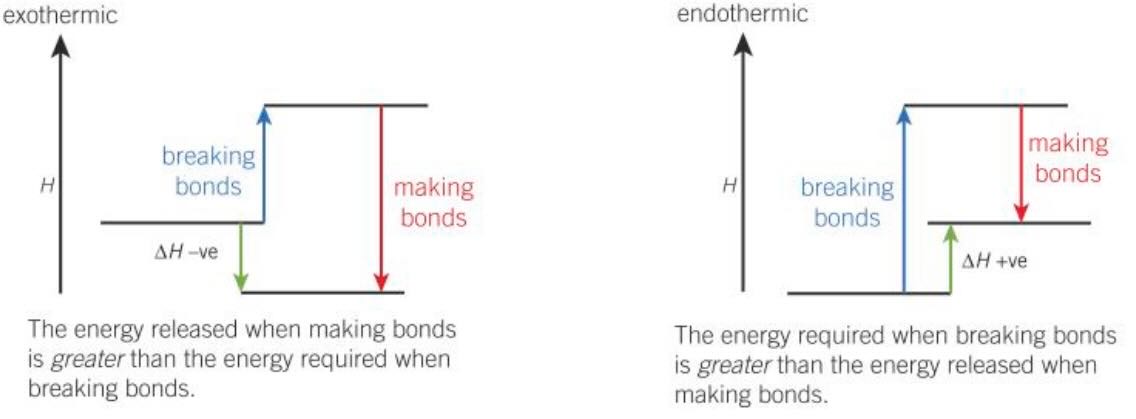
Endothermic / exothermic
- Endothermic
- Bond breaking is endothermic
- More energy required when breaking bonds than energy released when making bonds
- \(\Delta H > 0\)
- Exothermic
- Bond formation is exothermic
- More energy released when making bonds than energy required when breaking bonds
- \(\Delta H < 0\)
Calculating enthalpy change from average bond enthalpies
- \(\Delta_{r} H = \Sigma (\text{bond enthalpies in reactants}) - \Sigma (\text{bond enthalpies in products})\)
Working out enthalpy change using Hess's Law
- Using enthalpy change of formation: \(\Delta_{r} H = \Sigma \Delta_{f} H (\text{products}) - \Sigma \Delta_{f} H (\text{reactants})\)
- Using enthalpy change of combustion: \(\Delta_{r} H = \Sigma \Delta_{c} H (\text{reactants}) - \Sigma \Delta_{c} H (\text{products})\)