3.1.3 The Halogens
Physical properties
Trend in boiling points
- Boiling point increases down the group
- Halogens exist as diatomic molecules at RTP
- Number of electron shells in the atom increases going down the group
- The atom gets bigger + heavier
- Number of electrons in the diatomic molecules increases
- Stronger induced dipole-dipole interactions
- More energy required to break the intermolecular forces

Appearance under different states
| Halogen |
Colour in natural state |
Colour in aqueous solutions |
Colour in organic solvents |
| \(F_2\) |
Pale yellow gas |
/ |
/ |
| \(Cl_2\) |
Pale green gas |
Pale green |
Pale green |
| \(Br_2\) |
Red-brown liquid |
Orange |
Orange |
| \(I_2\) |
Shiny grey-black solid |
Brown |
Violet |
Redox reactions
Redox reaction of halogens
- Halogens all have \(s^2p^5\) electron configuration in their outer shell
- They gain 1 electron to form 1- ion during redox reactions and gain the electron configuration of the nearest noble gas (reduced)
- Another species loses electrons to halogen atoms so it is oxidised
- Halogens are oxidising agents as they oxidise other species
- Halide ions combine with metal ions to form white (ionic) solids which are mostly soluble
Halogen-halide displacement reactions
- Solution of halogen added to other halide solutions
- If the halogen added is more reactive than the halide ion in the solution
- It will displace the halide ion in the solution
- The solution changes colour (from ... to ...)
- Results
- \(Cl_2\) reacts with \(Br^-\) (\(Cl_2(aq) + 2Br^-(aq) \rightarrow 2Cl^-(aq) + Br_2(aq)\) orange)
- \(Cl_2\) reacts with \(I^-\) (\(Cl_2(aq) + 2I^-(aq) \rightarrow 2Cl^-(aq) + I_2(aq)\) violet)
- \(Br_2\) reacts with \(I^-\) only (\(Br_2(aq) + 2I^-(aq) \rightarrow 2Br^-(aq) + I_2(aq)\) violet)
- \(I_2\) doesn't react at all
- Element displaced can be identified by adding cyclohexane (non-polar solvent, dissolve more readily) + mix
- Use the colour of the top layer to identify the element being displaced
- Show reactivity: \(Cl_2 > Br_2 > I_2\)
Trend in reactivity
- Reactivity decreases down the group
- Atomic radius increases
- More inner shells so shielding increases
- Effect of increasing nuclear charge is outweighed by increasing atomic radius and shielding
- Less attraction between the nucleus and the outer shell
- Harder for elements to capture an electron from another species and form 1- ions
Disproportionation reactions
Disproportionation
- A redox reaction in which the same element is both oxidised and reduced
Chlorinating water

- Used in water treatment systems to kill harmful bacteria
- Bacteria killed by chloric(I) acid (\(HClO\)) and chlorate(I) ions (\(ClO^-\))
- Chloric(I) acid also acts as a weak bleach e.g. indicator paper will turn red then white
Benefits and risk of chlorinating water
- Benefits
- Kill bacteria in water treatment
- Reduces the risk of waterborne diseases
- Risks
- Hazards of toxic chlorine gas \(\rightarrow\) respiratory irritant in small concentrations, can be fatal if in large concentrations
- Formation of chlorinated hydrocarbons which is carcinogenic
Manufacturing bleach
- React chlorine with cold and dilute aqueous \(NaOH\) solution
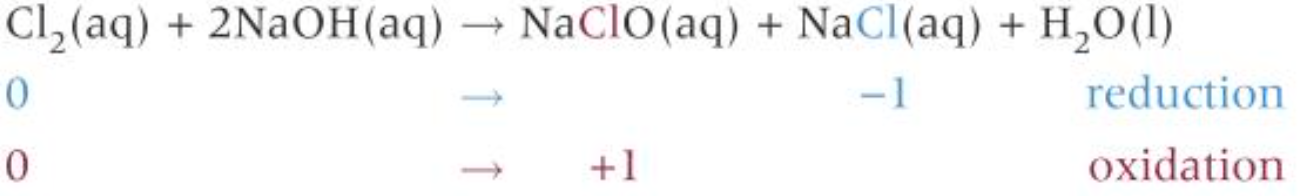
- \(NaClO\) solution = bleach
Halide test