3.1.1 Periodicity
Definitions
| Term | Definition |
|---|---|
| Periodicity | A repeating trend in physical and chemical properties of the elements across the periodic table. |
| Groups | A vertical column in the periodic table. Elements in a group have similar chemical properties and their atoms have the same number of outer shell electrons. |
| Periods | A horizontal row in the periodic table. Elements show trends in properties across a period. |
| Shielding effect | The repulsion between electrons in different inner shells. Shielding reduces the net attractive force between the positive nucleus and the outer shell electrons. |
| Metallic bonding | The strong electrostatic attraction between the regularly arranged metal cations and the delocalised valence electrons between them. |
| Delocalised electrons | Electrons shared between more than two atoms / ions. |
| Giant metallic lattice | A three dimensional structure of positive ions and delocalised electrons, bonded together by strong metallic bonds. |
| Giant covalent lattice | A three dimensional structure of atoms, bonded together by strong covalent bonds. |
The periodic table
History
- Then
- Mendeleev arranged the elements in order of atomic mass
- Swapped elements to arrange them into groups of similar properties
- Gaps left where he thought elements would be found
- Predicted properties for missing elements
- Newly discovered elements filled in the gap and matched the predicted properties
- Now
- Arranged in increasing atomic number
- In vertical columns (groups) with same number of outer electrons + similar properties and horizontal rows (periods) giving number of highest energy electron shell
Arrangement
- In the order of increasing atomic number
- Periodicity: in periods showing repeating trends in physical and chemical properties e.g. metals \(\rightarrow\) non-metals
- In groups with similar properties
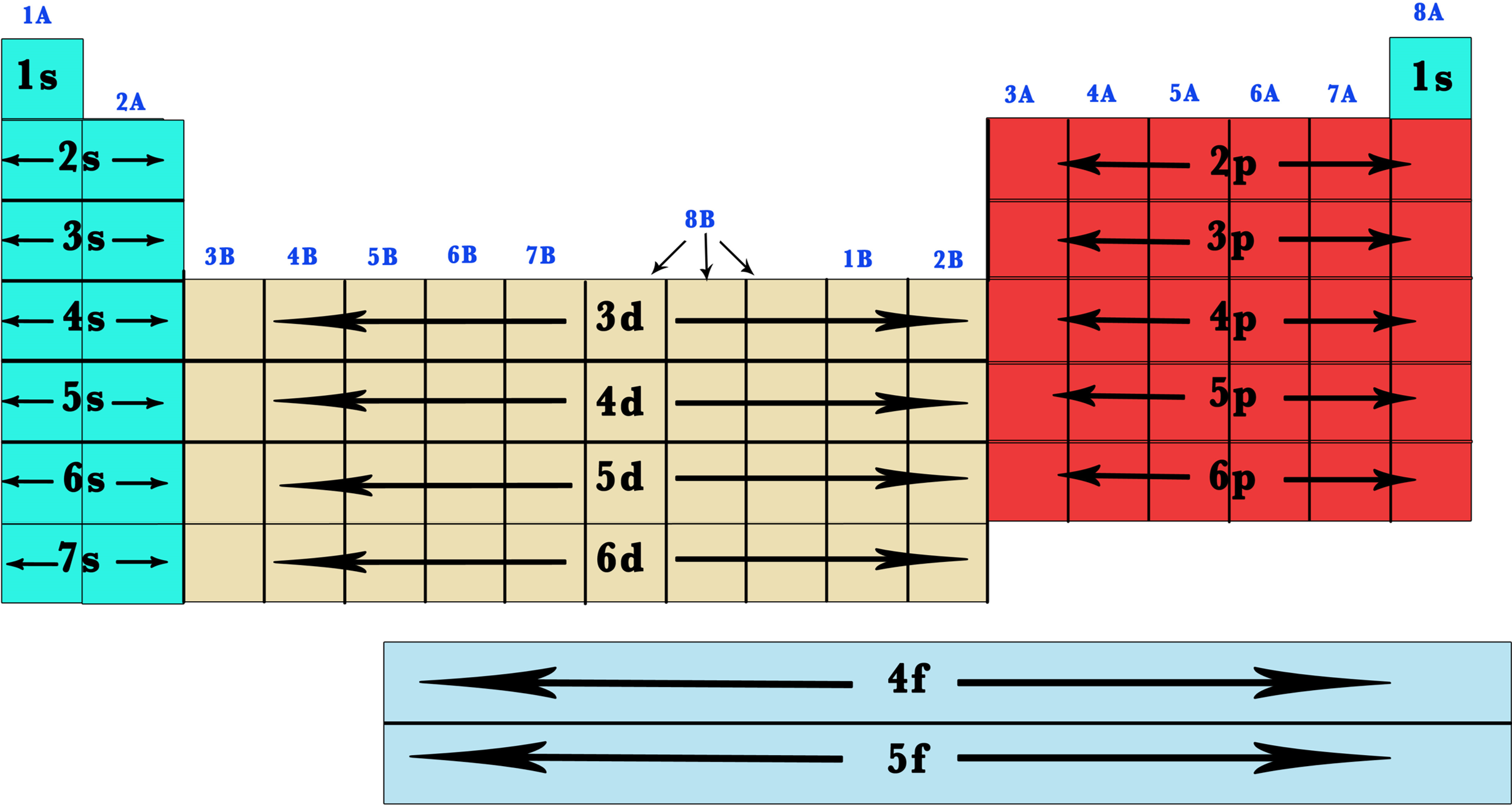
Electron configuration pattern
- Across period
- Each period starts with an electron in a new highest energy shell
- Period 2: \(2s\) fills \(\rightarrow\) \(2p\) fills
- Period 3: \(3s\) fills \(\rightarrow\) \(3p\) fills
- Period 4: only \(4s\) and \(4d\) occupied in shell
Blocks
- s/p/d/f-block meaning: the highest energy electron is in a s/p/d/f-orbital
- S, p, d and f block
Name of groups
| Group number | Name |
|---|---|
| 1 | Alkali metal |
| 2 | Alkaline earth metals |
| 3-12 | Transition elements |
| 15 | Pnictogens |
| 16 | Chalcogens |
| 17 | Halogens |
| 18 | Noble gases |
Ionisation energy
First ionisation energy
- Energy required to remove one electron from each atom in one mole of gaseous atoms of an element, forming one mole of gaseous 1+ ions
- Unit = \(kJ \cdot mol^{-1}\)
- Equation: \(X(g) \rightarrow X^+(g) + e^-\)
Factors affecting ionisation energy
- Atomic radius
- Greater distance between nucleus and outer electrons = less nuclear attraction
- Large effect on ionisation energy as force of attraction falls sharply with increasing distance
- Nuclear charge (weakest effect, outweighed)
- More protons in nucleus (greater nuclear charge) = greater attraction between the nucleus and the outer electrons = increase in ionisation energy
- Electron shielding
- Shielding effect: electrons are negatively charged so inner shell electrons repel outer-shell electrons
- Reduces the attraction between nucleus and outer electrons \(\rightarrow\) reduce ionisation energy
First ionisation energy trends across a period
- Increases across a period
- Nuclear charge increases
- Same number of shells so similar shielding
- Atomic radius decreases
- Nuclear attraction increases \(\rightarrow\) first ionisation energy increases
- Falls when the p sub-shell is starting to be filled (e.g. \(Li \rightarrow Be\))
- \(2p / 3p\) sub-shell has a higher energy than \(2s / 3s\) sub-shell so the electron is easier to remove
- Still larger than IE before the decrease
- Falls when pairing of electrons in p sub-shell starts (e.g. \(N \rightarrow O\))
- Paired electrons in one of the p orbitals repel one another so it is easier to remove an electron from the atom
- Still larger than IE before the decrease
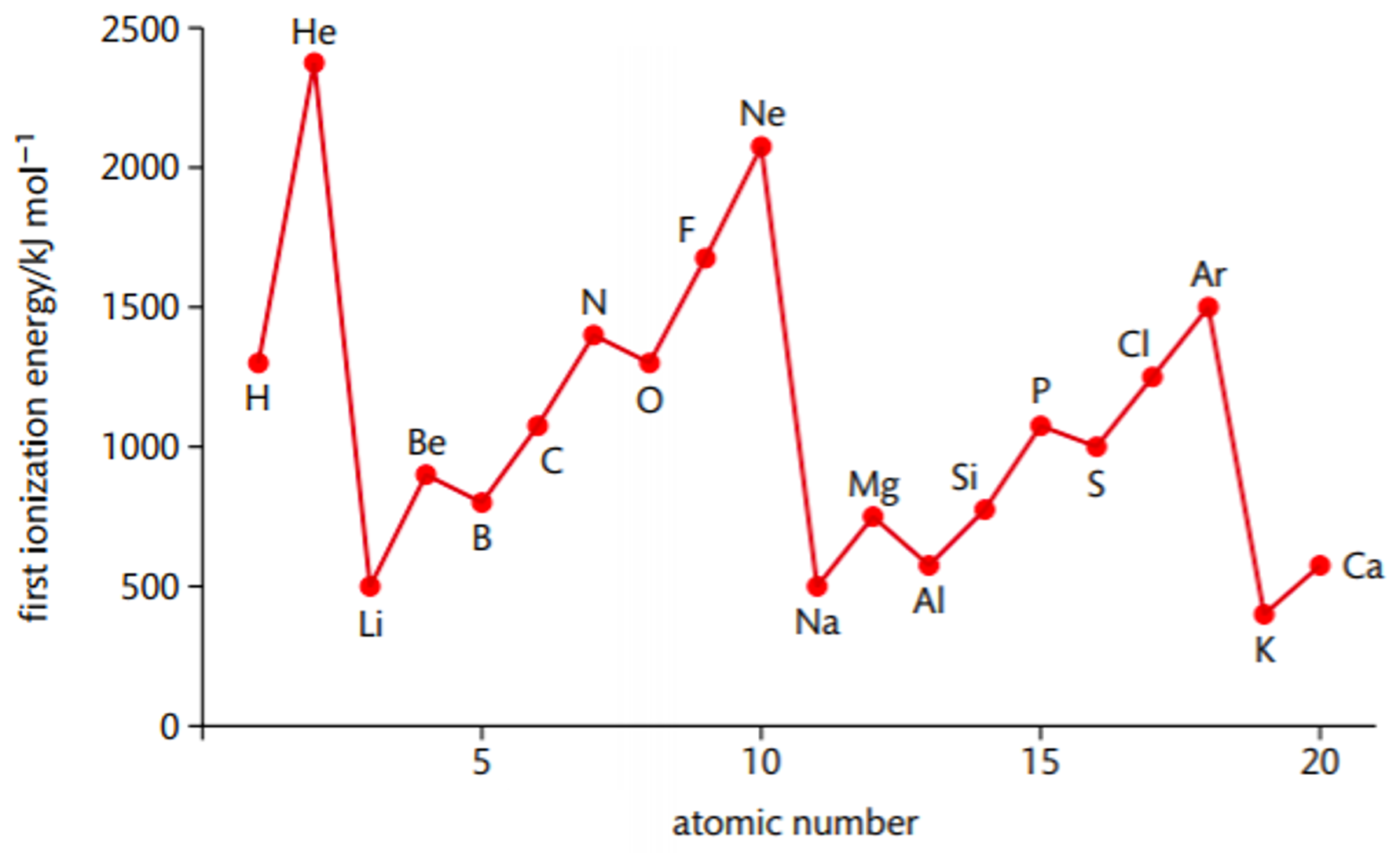
First ionisation energy trend down a group
- Decrease down a group
- Atomic radius increases
- More inner shells so shielding increases
- Increase in atomic radius and shielding outweighs the increasing nuclear charge
- Nuclear attraction on outer electrons decreases \(\rightarrow\) first ionisation energy decreases
Successive ionisation energy pattern
- Equation: e.g. \(Mg^+(g) \rightarrow Mg^{2+}(g) + e^-\)
- Larger than the previous one
- After the first electron is lost the remaining electrons are pulled closer to the nucleus
- Nuclear attraction on the remaining electrons increases so more energy needed
- Large increase when shell change
- Shell closer to the nucleus as atomic radius drops
- Less shielding present as there are now less inner shells between electron & nucleus
- Stronger nuclear attraction so more energy needed
- Going to a more inner shell = extremely large increase
- Smaller but still large increase when going to a new sub-shell / sub-shell become half filled
- Can be used to work out the number of electrons in each shell + group number of the element
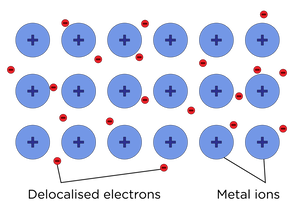
Metallic bond
Metallic bonding structure
- Regularly arranged metal cations sitting in a sea of delocalised electrons
- Each atom donate its negative outer shell electrons to a shared pool of electrons which are delocalised throughout the whole structure
- Cations left behind = nucleus + inner shell electrons
- Cations are fixed in position
- Delocalised electrons are mobile and free to move throughout the structure
- Forms a giant metallic lattice
Properties of metals
- All conduct electricity
- Delocalised electrons can move through the structure and carry charge through the structure when a voltage is applied across a metal
- More delocalised electrons \(\rightarrow\) more electrons can move \(\rightarrow\) better conductivity
- Conducts electricity both in solid state and when molten
- Most have high melting and boiling points
- Depends on the strength of metallic bonds
- Greater cation charge = stronger attractive forces as more electrons are delocalised and forces between electrons + cations are stronger
- Larger ions = weaker attractive forces due to larger atomic radius decreasing the charge density
- High temperature needed to provide the large amount of energy to overcome strong electrostatic attraction between the cations and the electrons
- Melting and boiling points decrease down the group
- Depends on the strength of metallic bonds
- Dissolve in liquid metals only
- Similar force between particles
- Any interaction between polar / non-polar solvent + solute lead to a reaction rather than dissolving
- Forces between particles are too large so it is not energetically favourable for them to mix
Giant covalent structures
Giant covalent structures
- Boron, carbon allotropes, and silicon (\(Si\), \(SiO_2\), \(SiC\))
- A network of atoms bonded by strong covalent bonds to form a giant covalent lattice
Diamond / silicon
- 4 outer shell electrons of each atom form 4 covalent bonds with other carbon / silicon atoms
- Tetrahedral structure
- 109.5° bond angle due to electron-pair repulsion
- High melting and boiling points
- Atoms held together by strong covalent bonds
- High temperatures are needed to provide the large quantity of energy needed to break the strong covalent bonds
- Non-conductors of electricity
- All 4 outer-shell electrons involved in covalent bond so no charged particles or mobile ions are available for conducting electricity
Graphite
- Flat 2D sheets of hexagonally arranged carbon atoms (trigonal planar 120°)
- Layers bonded by weak London forces \(\rightarrow\) they can slide over each other easily so graphite is soft
- High melting and boiling points
- Atoms held together by strong covalent bonds
- High temperatures are needed to provide the large quantity of energy needed to break the strong covalent bonds
- Can conduct electricity
- One electron from each carbon atom is delocalised and is available for conductivity
Graphene
- Single layer of graphite
- Hexagonally arranged (trigonal planar 120°) carbons
- Very hard as there are no points of weakness in the structure
- One of the thinnest + strongest material in existence (atoms held together by strong covalent bond)
- High melting and boiling points
- Atoms held together by strong covalent bonds
- High temperatures are needed to provide the large quantity of energy needed to break the strong covalent bonds
- Can conduct electricity
- One electron from each carbon atom is delocalised
- They can move and conduct electricity
Applications of graphene
- Electronics
- Flexible displays
- Wearables
- Other next-generation electronic devices
Periodic trends in properties
Atomic radii trend across a period
- Atomic radii decreases across the period
- Positive charge in nucleus and negative charge in the outer shell both increases
- Shielding remains similar as the number of shells doesn't change
- The attraction between the nucleus and the outer electrons increases
Melting / boiling point trend across a period
- Increases from Group 1 to 14
- Sharp decrease between Group 14 to 15 - change from giant to simple molecular structures
- Comparatively low from Group 15 to 18
- The exact boiling points depend on the type of covalent bonding
- Giant covalent bonding = very high melting and boiling points
- Simple covalent bonding = depends on strength of intermolecular forces (London forces) which depends on the mass of the nucleus
- Smaller molecular radius = lower boiling points (hence Group 18 has the lowest boiling points)