2.2.2 Bonding and structure
Definitions
| Term | Definition |
|---|---|
| Polarity | There is an uneven distribution of electrons. |
| Polar molecule | A molecule with an overall dipole, having taken into account any dipole across bonds and the shape of the molecule. |
| Bond angle | The angle between 2 bonds in a molecule. |
| Electronegativity | A measure of the attraction of a bonded atom for the shared pair of electrons in a covalent bond. |
| Dipole | A separation in electrical charge so that one atom of a polar covalent bond, or one end of a polar molecule, has a small positive charge, \(\delta+\), and the other has a small negative charge, \(\delta-\). |
| Intermolecular forces | An attractive force between molecules. Can be London forces, permanent dipole-dipole interactions or hydrogen bonding. |
| Giant ionic lattice | A three dimensional structure of oppositely charged ions, bonded together by strong ionic bonds. |
| Simple molecular lattice | A three dimensional structure of molecules, bonded together by weak intermolecular forces. |
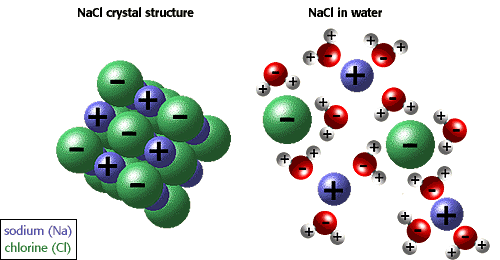
Ionic compounds
Ionic bonding
- The strong electrostatic attraction between positive and negative ions
- Occurs between atoms that have a big difference in electronegativity (usually metals and non-metals)
Dot and cross diagram
Structure of ionic compounds
- Each ion attracts oppositely charged ions strongly in all directions
- Forms a giant lattice structure containing many ions
- Every positive ion is surrounded by negative ions; every negative ion is surrounded by positive ions
- Regular arrangement of ions = regular, predictable shapes depending on size of ions
- \(NaCl\) = always cubic
- \(MgSO_4\), \(CuSO_4\), etc. = not cubic

Properties
- High melting and boiling points
- Strong electrostatic forces of attraction between oppositely charged ions in the giant ionic lattice
- A high temperature is needed to provide the large amount of energy needed to overcome the strong electrostatic attraction
- Higher for ions with greater ionic charges due to stronger attraction between ions
- Ionic substances with complex ions: can decompose before they reach melting point
- Dissolve in polar solvents (e.g. water)
- Solvation / hydration
- Polar solvent molecules attract the ions in the lattice
- Anions to positive dipole, cations to negative dipole
- Energy is released in the process
- Molecules break down the lattice + surround each ion in solution
- Strong ionic bond must be overcome for the substance to dissolve
- Energy to do this is supplied by solvating / hydrating the ions / absorbed from the surroundings
- If the compound is made of ions with strong charges the attraction can be too strong to be broken down \(\rightarrow\) less soluble
- Solubility depends on
- Relative strengths of attractions within the lattice
- Attraction between ions + water molecules
- Solvation / hydration
- Does not conduct electricity in solid state
- Ions held in fixed positions
- No mobile charge carriers
- Conduct electricity when dissolved or molten
- The solid ionic lattice breaks down
- Ions are free to move as mobile charge carriers
Covalent compounds
Covalent bonding
- The strong electrostatic attraction between a shared pair of electrons and the nuclei of the bonded atoms
- Occur between atoms that have a small difference in electronegativity
- Formed when the atomics orbitals of the two atoms overlap and combine to form a new orbital (still holding 2 electrons max)
Covalent substance structure
- Attraction is localised between the shared pair of electrons and the nuclei of the bonded atoms only
- Results in small molecules consisting two or more atoms (simple molecular lattice if solid)
Lone pairs / non-bonding pair
- Paired electrons that are not shared
Multiple covalent bond
- The electrostatic attraction is between two / three shared pairs of electrons and the nuclei of the bonded atoms
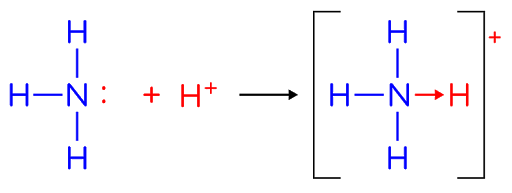
Dative covalent bonds / coordinate bonds
- The shared pair of electrons is supplied by one of the bonding atoms only
- The shared pair is originally a lone pair of electrons on one of the bonded atoms
- e.g. ammonia molecule donates its lone pair of electrons to a \(H^+\) ion to form \(NH_4^+\) ion
 |
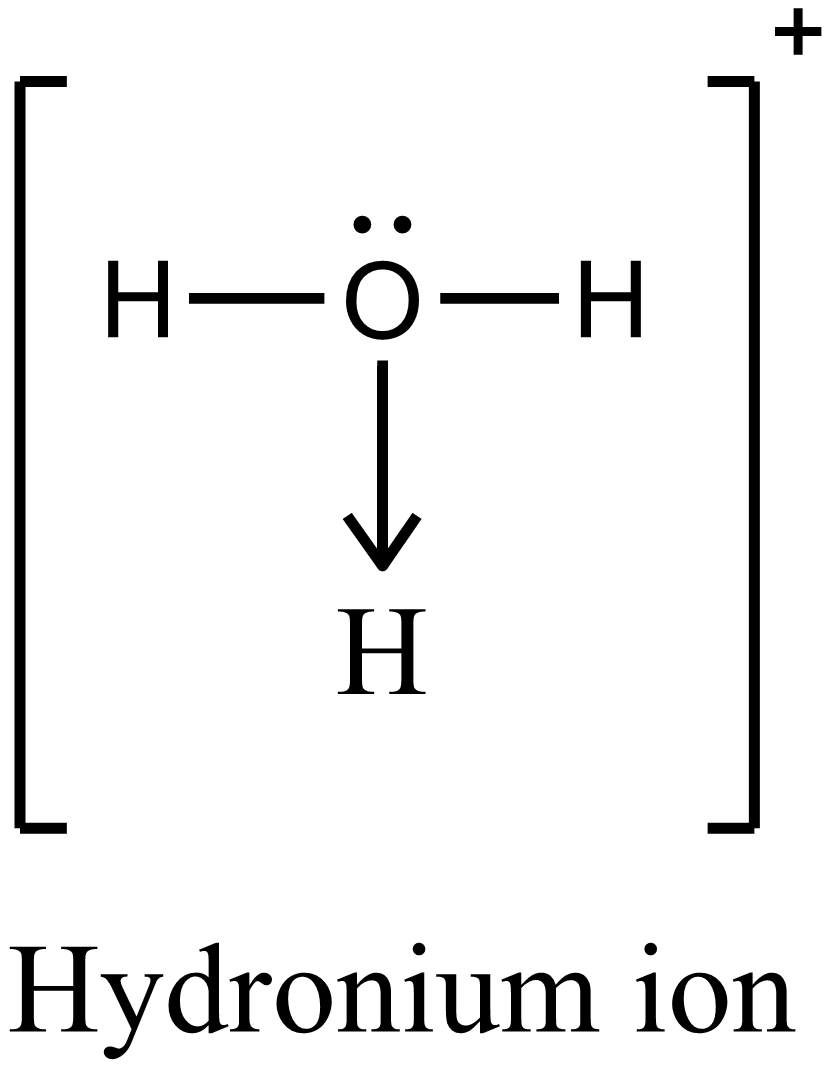 |
Exceptions
- Boron
- Only 3 outer shell electrons can be paired \(\rightarrow\) only forms 3 pairs
- e.g. \(BF_3\) only has 6 electrons around the boron atom
- Phosphorus, sulfur, chlorine (expansion of the octet)
- Outer electrons are in shell 3 which can hold up to 18 electrons
- More electrons are available for bonding (can have more than 8 electrons in outer shell e.g. \(ClF_7\))
Average bond enthalpy
- A measurement of covalent bond strength
- Higher average bond enthalpy = stronger covalent bond
Molecule structures
Molecule shape and bond angles
- Electron pairs around the central atom repel each other as far apart as possible
- Lone pairs repel more strongly than bonded pairs
- They repel bonded pairs slightly closer together \(\rightarrow\) reducing the bond angle
- 4 pairs / regions: bond angle reduced by approx. 2.5° per lone pair
- The greater the number of electron pairs the smaller the bond angle
- (Mention the number of bonded pairs and lone pairs)
| Electron pairs | Bonded pairs | Lone pairs | Name | Shape and bond angle |
|---|---|---|---|---|
| 2 | 2 | 0 | Linear | 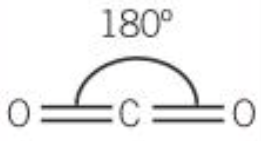 |
| 3 | 3 | 0 | Trigonal planar | 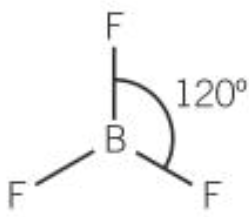 |
| 4 | 4 | 0 | Tetrahedral | 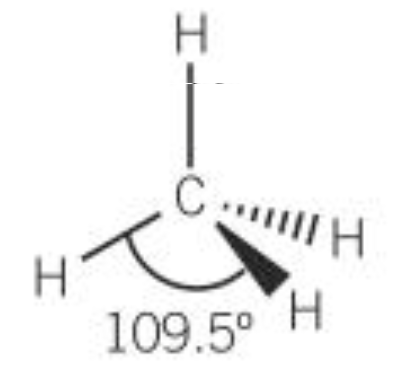 |
| 4 | 3 | 1 | Pyramidal | 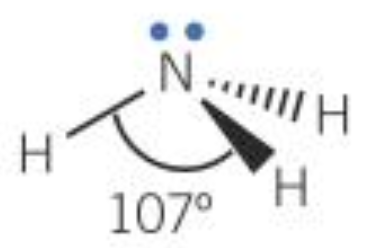 |
| 4 | 2 | 2 | Non-linear | 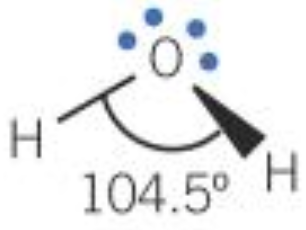 |
| 6 | 6 | 0 | Octahedral | 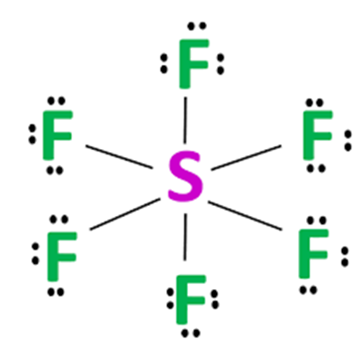 Remember \(SF_6\) as an example of octahedral arrangement |
Bond representations
Predicting molecular shape of ions
- Ions with oxygen
- Add an electron to an oxygen atom for every negative charge
- Bonds can be single or double bond
- Cations with hydrogen
- Add positive charge by \(H^+\) ions
- Others
- Add / remove electrons from the central atom to match the charge
- Pair up surrounding atoms and count bonded pairs and lone pairs
Electronegativity and bond polarity
Electronegativity
- A measure of the attraction of a bonded atom for the pair of electrons in a covalent bond
- Measured using Pauling electronegativity values (higher = attract bonded electrons more strongly)
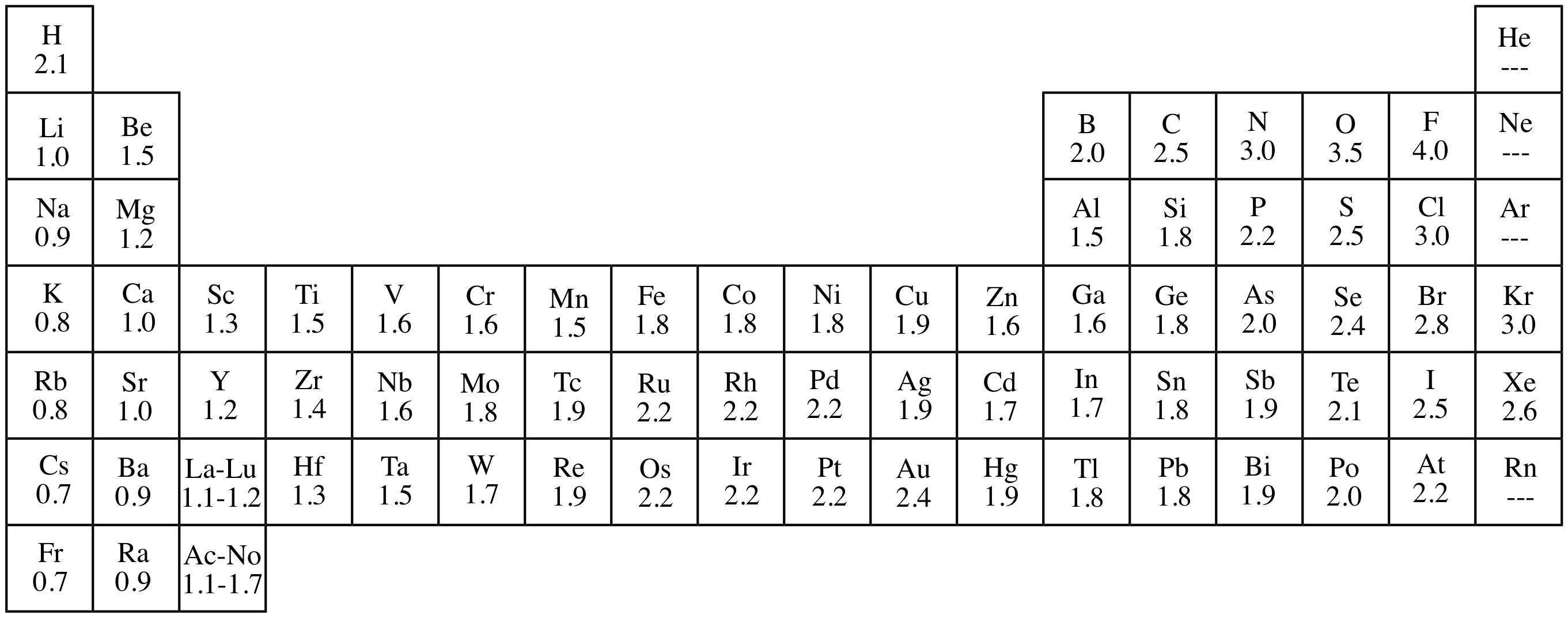
Important electronegativity values
| Element | Electronegativity |
|---|---|
| Fluorine | 4.0 |
| Oxygen | 3.5 |
| Chlorine | 3.0 |
| Nitrogen | 3.0 |
| Carbon | 2.5 |
| Hydrogen | 2.1 |
Electronegativity pattern
- Electronegativity increases across the periodic table and up the periodic table
- Across the periodic table the nuclear charge increases and the atomic radius decreases \(\rightarrow\) attraction increases
- Atomic radius increases down the table + increased shielding \(\rightarrow\) attraction decreases
- Fluorine is the most electronegative element
Predicting type of bond
| Bond type | Electronegativity difference |
|---|---|
| Covalent | 0 |
| Polar covalent | 0 - 1.8 |
| Ionic | > 1.8 |
Pure covalent bond
- Non-polar bond
- The bonded electron pair shared equally between the bonded atoms
- Happen when:
- The bonded atoms are the same
- The bonded atoms have same / similar electronegativity (e.g. carbon and hydrogen)
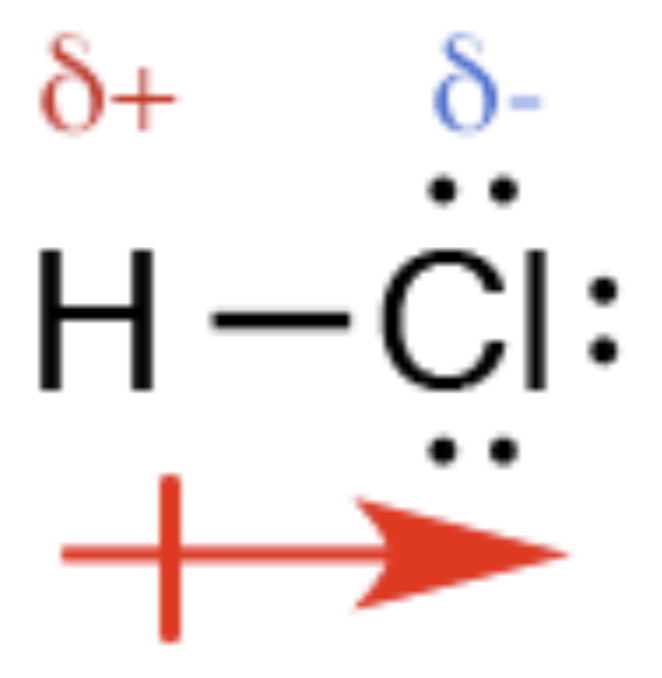
Polar covalent bonds
- Polar bond
- The bonded electron pair is shared unequally between the bonded atom
- When the bonded atoms are different and have different electronegativity values
- Form permanent dipole: small positive (\(\delta+\)) / negative charge (\(\delta-\)) on the two bonded atoms, oppositely charged on each
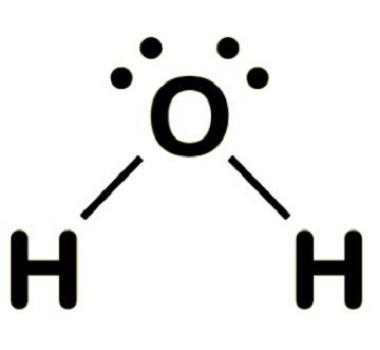
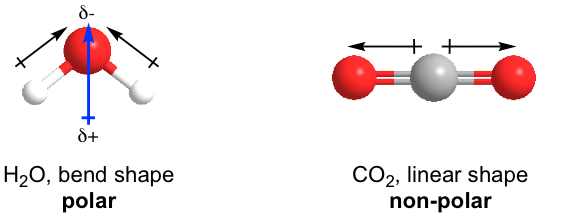
Polar / non-polar molecules
- Polar molecules require polar bonds with dipoles that do not cancel out due to their direction
- If the molecule is symmetrical in 3D and the polarities cancel out the molecule is non-polar
- e.g. overall dipole in \(H_2O\), cancels out in \(CO_2\) and methane
Intermolecular forces
Types of intermolecular forces
- London forces / induced dipole-dipole interactions / dispersion forces (van der Waals' forces)
- Permanent dipole-dipole interactions (van der Waals' forces)
- Hydrogen bonding (a special type of permanent dipole-dipole interactions)
London forces / induced dipole-dipole interactions
- Exist between all molecules, only temporary
- Creation
- Movement of electrons produces changing dipole in a molecule
- There is an uneven distribution of electrons which causes an instantaneous dipole
- At any instant an instantaneous dipole will exist but position shifts constantly
- Instantaneous dipole induces a dipole on a neighbouring molecule
- The induced dipole induces dipoles on further molecules, they then attract one another
- Electrons move and the dipole is gone and the attraction disappears and re-appears elsewhere
- Very weak - the weakest of all intermolecular forces
- The only intermolecular force that exist between non-polar simple covalent molecules
- They have very low melting and boiling points (normally gases / volatile liquids under room temperature)
London forces strength pattern
- Stronger down the periodic table
- More electrons in each molecule
- Larger instantaneous + induced dipoles
- Greater induced dipole-dipole interactions
- Stronger electrostatic attractive forces between molecules
Permanent dipole-dipole interactions
- Act between permanent dipoles in polar molecules
- Permanent dipole-dipole interactions is much stronger than London forces
- Polar molecules have both London forces and permanent dipole-dipole interactions
- Mean melting and boiling point of polar molecules is much greater
Hydrogen bond
- Occurs when a highly de-shielded hydrogen in one molecule is attracted to a lone pair on a N, O or F atom in another molecule
- Highly de-shielded hydrogen: a hydrogen atom directly bonded to an very electronegative atom (N, O or F)
- A lone pair of electrons on a N/O/F atom forms bond with the \(\delta+\) of a hydrogen atom in a different molecule
- Strongest type of intermolecular attractions
- Molecules with hydrogen bonds between them have relatively high melting and boiling points
- More hydrogen bond =