2.2.1 Electron structure
Definitions
| Term | Definition |
|---|---|
| Atomic orbitals | A region around the nucleus that can hold up to two electrons with opposite spins. |
| Sub-shells | A group of orbitals of the same type within a shell. |
| Electron configuration | A shorthand method for showing how electrons occupy sub-shells in an atom. |
Atomic orbitals
Number of electrons that can fit in each shell
| Shell number | Number of electrons ( \(= 2n^{2}\) ) |
|---|---|
| 1 | 2 |
| 2 | 8 |
| 3 | 18 |
| 4 | 32 |
Types of orbitals
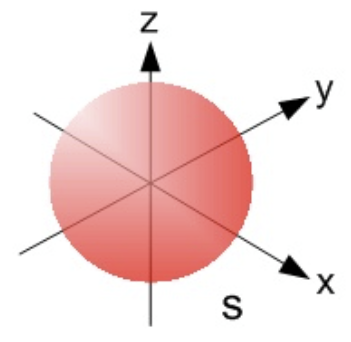
- s-orbitals
- Spherical shape
- Each shell from \(n=1\) contains 1 s-orbital
- Greater shell number \(n\) = greater radius of its s-orbital
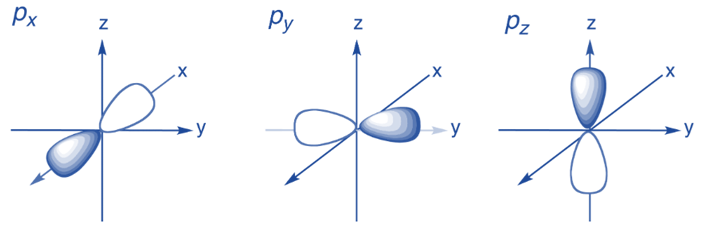
- p-orbitals
- Dumb-bell shape
- Three separate p-orbitals at right angles to one another: \(p_x, p_y, p_z\)
- Each shell from \(n=2\) contains 3 p-orbitals
- Greater shell number \(n\) = its p-orbital is further from the nucleus
- d-orbitals
- Each shell from \(n=3\) contains 5 d-orbitals
- f-orbitals
- Each shell from \(n=4\) contains 7 f-orbitals
Filling of sub shells and orbitals
- Sub shells fill in the order of increasing energy (\(1s \rightarrow 2s \rightarrow 2p \dots\))
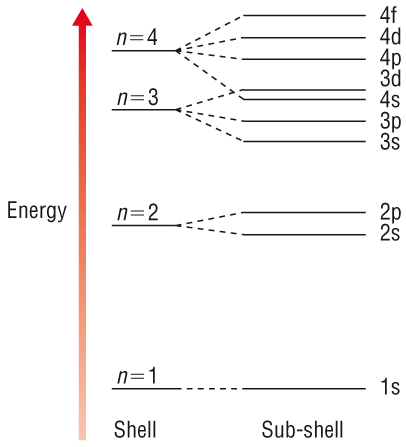
- \(4s\) is filled before \(3d\)
- Inside each sub-shell
- The orbitals all have the same energy within a sub-shell
- One electron occupies each orbital before pairing starts to prevent any repulsion between paired electrons
- Opposite spins within each orbital (one \(\uparrow\) and one \(\downarrow\)) as both electrons are negatively charged and repel one another so this minimises repulsion
- The paired electrons are easier to remove than the unpaired ones
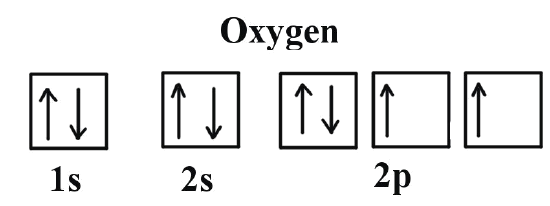
- More than one orbital within a sub-shell = the orbitals are degenerate (all the same)
Electron configurations
Writing electron configuration of atoms
- e.g. \(Li = 1s^2 2s^1\), \(F = 1s^2 2s^2 2p^5\)
- Shorthand notation: in terms of the previous noble gas + outer electron sub-shells
- e.g. Krypton = \(1s^2 2s^2 2p^6 3s^2 3p^6 3d^{10} 4s^2 4p^6\) === \([Ar] 3d^{10} 4s^2 4p^6\)
- Always show outer shell electrons
- (4s can be written before 3d)
Exceptions of atom electron configurations
- Chromium: \([Ar] 3d^5 4s^1\)
- Copper: \([Ar] 3d^{10} 4s^1\)
- Chromium and copper do not follow the expected pattern
- Half-filled / fully filled d sub-shell gives additional stability
Electron configuration of ions
- The highest energy subshell gain or lose electrons
- d-block elements
- \(4s\) is at a lower energy level than \(3d\) sub-shell so it is filled first
- Once filled the energy level of \(3d\) falls below \(4s\) so \(4s\) empties before \(3d\)